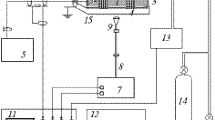
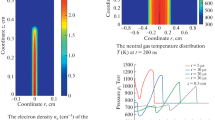
Abstract—
Studies of the composition of the products of chemical reactions in the afterglow of a new type of atmospheric discharge, the microwave self-non-self-sustained discharge in air with admixtures of methane, were carried out. A detailed kinetic model was developed that describes the kinetics of reactions with polyatomic nitrogen oxides at the stage of intensive cooling of the discharge channel. It was found that the formation of nitrogen oxide NO depends on the percentage of the products of the methane molecule dissociation in the nitrogen–oxygen mixture that, initially, is atomic. The new discharge type can be used for the plasmachemical processing of gaseous media.



Similar content being viewed by others
REFERENCES
G. M. Batanov, S. I. Gritsinin, I. A. Kossyi, A. N. Magunov, V. P. Silakov, and N. M. Tarasova, in Plasma Physics and Plasma Electronics, Ed. by L. M. Kovrizhnykh (Nauka, Moscow, 1985; Nova Science, Commack, NY, 1985).
G. M. Batanov, S. I. Gritsinin, and I. A. Kossyi, J. Phys. D: Appl. Phys. 35, 2687 (2002).
I. A. Kossyi, in Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, 2006, Paper AIAA-2006-1457.
G. M. Batanov, N. K. Berezhetskaya, A. M. Davydov, E. M. Konchekov, I. N. Katorgin, I. A. Kossyi, K. A. Sarksyan, V. D. Stepakhin, S. M. Temchin, and N. K. Kharchev, Prikl. Fiz., No. 5, 10 (2017).
K. V. Artem’ev, G. M. Batanov, N. K. Berezhetskaya, A. M. Davydov, I. A. Kossyi, V. I. Nefedov, K. A. Sarksyan, and N. K. Kharchev, Usp. Prikl. Fiz. 5, 429 (2017).
K. V. Artem’ev, G. M. Batanov, N. K. Berezhetskaya, A. M. Davydov, L. V. Kolik, E. M. Konchekov, I. A. Kossyi, A. E. Petrov, K. A. Sarksyan, V. D. Stepakhin, and N. K. Kharchev, Plasma Phys. Rep. 44, 1146 (2018).
K. V. Artem’ev, G. M. Batanov, N. K. Berezhetskaya, V. D. Borzosekov, S. I. Gritsinin, A. M. Davydov, L. V. Kolik, E. M. Konchekov, I. A. Kossyi, Yu. A. Lebedev, I. V. Moryakov, A. E. Petrov, K. A. Sarksyan, V. D. Stepakhin, N. K. Kharchev, et al., Plasma Phys. Rep. 46, 311 (2020).
Yu. P. Raizer, Gas Discharge Physics (Nauka, Moscow, 1987; Springer-Verlag, Berlin, 1991).
V. K. Zhivotov, B. V. Potapkin, and V. D. Rusanov, in Encyclopedia of Low-Temperature Plasma, Ed. by V. E. Fortov, Ser. B, Vol. VIII-1: Chemistry of Low-Temperature Plasma, Ed. by Yu. A. Lebedev, N. A. Plate, and V. E. Fortov (Yanus-K, Moscow, 2005), p. 4 [in Russian].
A. S. Zarin, A. A. Kuzovnikov, and V. M. Shibkov, Freely Localized Microwave Discharge in Air (Neft’ i Gaz, Moscow, 1996) [in Russian].
Yu. A. Lebedev, Plasma Sources Sci. Technol. 24, 053001 (2015).
K. V. Aleksandrov, L. P. Grachev, I. I. Esakov, S. M. Pokras, and K. V. Khodataev, Tech. Phys. 48, 43 (2003).
A. L. Vikharev and O. A. Ivanov, in Encyclopedia of Low-Temperature Plasma, Ed. by V. E. Fortov, Ser. B, Vol. VIII-1: Chemistry of Low-Temperature Plasma, Ed. by Yu. A. Lebedev, N. A. Plate, and V. E. Fortov (Yanus-K, Moscow, 2005), p. 356 [in Russian].
Yu. A. Lebedev, in Encyclopedia of Low-Temperature Plasma, Ed. by V. E. Fortov, Ser. B, Vol. VIII-1: Chemistry of Low-Temperature Plasma, Ed. by Yu. A. Lebedev, N. A. Plate, and V. E. Fortov (Yanus-K, Moscow, 2005), p. 435 [in Russian].
A. V. Kim and G. M. Fraiman, Sov. J. Plasma Phys. 9, 358 (1983).
I. A. Kossyi, A. Yu. Kostinsky, A. A. Matveyev, and V. P. Silakov, Plasma Sources Sci. Technol. 1, 207 (1992).
R. Kh. Amirov and E. A. Filimonova, in Encyclopedia of Low-Temperature Plasma, Ed. by V. E. Fortov, Ser. B, Vol. VIII-1: Chemistry of Low-Temperature Plasma, Ed. by Yu. A. Lebedev, N. A. Plate, and V. E. Fortov (Yanus-K, Moscow, 2005), p. 463 [in Russian].
R. Kh. Amirov and E. A. Filimonova, in Encyclopedia of Low-Temperature Plasma, Ed. by V. E. Fortov, Ser. B, Vol. VIII-1: Chemistry of Low-Temperature Plasma, Ed. by Yu. A. Lebedev, N. A. Plate, and V. E. Fortov (Yanus-K, Moscow, 2005), p. 502 [in Russian].
L. V. Gurvich, I. V. Veits, B. A. Medvedev, G. A. Khachkuruzov, V. S. Yungman, G. A. Bergman, V. F. Baibuz, V. S. Iorish, G. N. Yurkov, S. I. Gorbov, L. F. Kuratova, N. P. Rtishcheva, I. N. Przheval’skii, V. Yu. Zitserman, V. Ya. Leonidov, et al., Thermodynamic Properties of Individual Substances. Reference Book (Nauka, Moscow, 1978–1982), Vols. 1−4 [in Russian].
M. Capitelli, C. M. Ferreira, B. F. Gordiets, and A. I. Osipov, Plasma Kinetics in Atmospheric Gases (Springer-Verlag, Berlin, 2000).
B. F. Gordiets, C. M. Ferreira, V. L. Guerra, J. Loureiro, J. Nahormy, D. Pagnon, M. Touzeau, and M. Vialle, IEEE Trans. Plasma Sci. 23, 750 (1995).
N. A. Popov, Doctoral Dissertation in Physics and Mathematics (Skobeltsyn Institute of Nuclear Physics of Moscow State University, Moscow, 2009).
N. L. Aleksandrov, F. I. Vysikailo, R. Sh. Islamov, I. V. Kochetov, A. P. Napartovich, and V. G. Pevgov, High Temp. 19, 342 (1981).
Yu. S. Akishev, A. A. Deryugin, V. B. Karal’nik, I. V. Kochetov, A. P. Napartovich, and N. I. Trushkin, Plasma Phys. Rep. 20, 511 (1994).
I. V. Kochetov, E. V. Mishin, and V. A. Telegin, Sov. Phys.–Dokl. 31, 990 (1986).
N. G. Dautov and A. M. Starik, Kinet. Katal. 38 (2), 207 (1997).
L. S. Polak, M. Ya. Gol’denberg, and A. A. Levitskii, Computational Methods in Chemical Kinetics (Nauka, Moscow, 1984) [in Russian].
Yu. K. Karasevich, Doctoral Dissertation in Physics and Mathematics (Semenov Institute of Chemical Physics, Moscow, 2007).
N. M. Marinov, W. J. Pitz, C. K. Westbrook, M. Hori, and N. Matsunaga, Proc. Combust. Inst. 27, 389 (1998).
Nonequillibrium Physicochemical Processes in Gas Flows and New Principles of Combustion Organization, Ed. by A. M. Starik (Torus, Moscow, 2011) [in Russian].
V. P. Agafonov, V. K. Vertushkin, A. A. Gladkov, and O. Yu. Polyanskii, Nonequillibrium Physicochemical Processes in Aerodynamics (Mashinostroenie, Moscow, 1972) [in Russian].
O. E. Krivonosova, S. A. Losev, V. P. Nalivaiko, Yu. K. Mukoseev, and O. P. Shatalov, in Plasma Chemistry, Ed. by B. M. Smirnov (Energoatomizdat, Moscow, 1987), Vol. 14, p. 3 [in Russian].
Handbook of Physiochemical Processes in Gas Dynamics, Vol. 1: Dynamics of Physiochemical Processes in Gases and Plasmas, Ed. by G. G. Chernyi and S. A. Losev (Mosk. Gos. Univ., Moscow, 1995) [in Russian].
Handbook of Physiochemical Processes in Gas Dynamics, Vol. 2: Physicochemical Kinetics and Thermodynamics, Ed. by G. G. Chernyi and S. A. Losev (Scientific Publishing Center of Mechanics, Moscow, 2002) [in Russian].
N. G. Dautov and A. M. Starik, High Temp. 31, 253 (1993).
D. J. Kewley and H. G. Hornung, Chem. Phys. Lett. 25, 531 (1974).
RRATE Database on Chemical Kinetics (AVOGADRO Center, Institute of Mechanics, Moscow State University, Moscow, 1992).
J. Heimerl and T. P. Coffee, Combust. Flame 35, 117 (1979).
S. Toby and E. Ullrich, Int. J. Chem. Kinet. 12, 535 (1980).
D. L. Baulch, D. D. Drysdale, J. Duxbary, and S. L. Grant, Evaluated Kinetic Data for High Temperature Reactions, Vol. 3: Homogeneous Gas Phase Reactions of O 2 –O 3 Systems, the CO–O 2 –H 2 System and of Sulphur-Containing Species (Butterworths, London, 1976).
D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr, J. Troe, and R. T. Watson, J. Phys. Chem. Ref. Data 13, 1259 (1984).
D. L. Baulch, D. D. Drysdale, and D. C. Horne, Evaluated Kinetic Data for High Temperature Reactions, Vol. 2: Homogeneous Gas Phase Reactions of the H 2 –N 2 –O 2 System (Butterworths, London, 1973).
K. L. Wray and J. D. Teare, J. Chem. Phys. 36, 2582 (1962).
M. Koshi, S. Bando, M. Saito, and T. Asaba, in Proceedings of the 17th Symposium (International) on Combustion, Pittsburgh, 1979, Symp. (Int.) Combust., [Proc.] 17, 553 (1979).
D. L. Baulch, R. A. Cox, P. J. Crutzen, R. F. Hampson, J. A. Kerr, J. Troe, and R. T. Watson, J. Phys. Chem. Ref. Data 11, 327 (1982).
S. W. Benson, D. Golden, R. W. Lawrence, R. Shaw, and R. W. Woolfolk, in Proceedings of the Symposium on Chemical Kinetics Data for the Upper and Lower Atmosphere, Warrenton, 1974, Int. J. Chem. Kinet.: Symp., No. 1, 399 (1975).
I. S. Zaslonko and Yu. K. Mukoseev, Khim. Fiz., No. 11, 1508 (1982).
J. Troe, Ber. Bunsen-Ges. Phys. Chem. 73, 906 (1969).
L. F. Phillips and H. I. Schiff, J. Chem. Phys. 42, 3171 (1965).
Y. Ono and M. A. A. Cline, Chem. Phys. 69, 381 (1982).
M. J. McEwan and L. F. Phillips, Chemistry of the Atmosphere (Halsted, New York, 1975).
Yu. K. Mukoseev and I. S. Zaslonko, Khim. Fiz., No. 1, 66 (1983).
D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr, J. Troe, and R. T. Watson, J. Phys. Chem. Ref. Data 13, 1259 (1984).
D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr, J. Troe, and R. T. Watson, J. Phys. Chem. Ref. Data 9, 295 (1980).
J. Troe, J. Chem. Phys. 66, 4758 (1977).
S. Mukkavilli, C. K. Lee, K. Varghese, and L. L. Tavlarides, IEEE Trans. Plasma Sci. 16, 652 (1988).
R. A. Graham and H. S. Johnston, J. Phys. Chem. 82, 254 (1978).
L. G. Piper, R. Krech, and R. L. Taylor, J. Chem. Phys. 75, 2099 (1979).
M. J. McEwan and L. F. Phillips, Chemistry of the Atmosphere (Halsted, New York, 1975).
A. A. Gvozdev, V. B. Nesterenko, G. V. Nichipor, and V. V. Trubnikov, Vestsi Akad. Navuk B. SSR, Ser. Fiz.-Tekh. Navuk, No. 2, 68 (1979).
I. S. Zaslonko, A. M. Tereza, O. N. Kulish, and D. Yu. Zhukov, Khim. Fiz., No. 11, 1491 (1992).
W. Tsang and R. F. Hampson, J. Phys. Chem. Ref. Data 15, 1087 (1986).
D. A. Masten, R. K. Hanson, and C. T. Bowman, J. Phys. Chem. 94, 7119 (1990).
R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson, Jr., J. A. Kerr, and J. Troe, J. Phys. Chem. Ref. Data 21, 1125 (1992).
M. W. Markus, D. Woiki, and P. Roth, in Proceedings of the 24th Symposium (International) on Combustion, Pittsburgh, 1992, Symp. (Int.) Combust., [Proc.] 24, 581 (1992).
M. Röhrig, E. L. Petersen, D. F. Davidson, R. K. Hanson, and C. T. Bowman, Int. J. Chem. Kinet. 29, 781 (1997).
D. L. Baulch, C. J. Cobos, R. A. Cox, P. Frank, G. Hayman, Th. Just, J. A. Kerr, T. Murrells, M. J. Pilling, J. Troe, R. W. Walker, and J. Warnatz, J. Phys. Chem. Ref. Data 23, 847 (1994).
A. Fahr, A. Laufer, R. Klein, and W. Braun, J. Phys. Chem. 95, 3218 (1991).
H. Wang and M. Frenklach, Combust. Flame 110, 173 (1997).
A. G. Zborovskii, Yu. P. Petrov, A. M. Tereza, and A. N. Tyurin, Khim. Fiz., No. 5, 1582 (1990).
R. D. Wilk, N. P. Cernansky, W. J. Pitz, and C. K. Westbrook, Combust. Flame 77, 145 (1989).
Y. Hidaka, T. Taniguchi, H. Tanaka, T. Kamesawa, K. Inami, and H. Kawano, Combust. Flame 92, 365 (1993).
R. S. Timonen, E. Ratajczak, D. Gutman, and A. F. Wagner, J. Phys. Chem. 91, 5325 (1987).
M. Klatt, M. Röhrig, and H. Gg. Wagner, Ber. Bunsen-Ges. Phys. Chem. 95, 1163 (1991).
A. M. Dean and P. R. Westmoreland, Int. J. Chem. Kinet. 19, 207 (1987).
F. Glarborg, J. A. Miller, and R. J. Kee, Combust. Flame 65, 177 (1986).
Y. Hidaka, T. Oki, H. Kawano, and T. Higashihara, J. Phys. Chem. 93, 7134 (1989).
W. Tsang, J. Phys. Chem. Ref. Data 16, 471 (1987).
Y. Hidaka, T. Nishimori, K. Sato, Y. Henmi, R. Okuda, K. Inami, and T. Higashihara, Combust. Flame 117, 755 (1999).
J. A. Miller and C. F. Melius, in Proceedings of the 22nd Symposium (International) on Combustion, Pittsburgh, 1989, Symp. (Int.) Combust., [Proc.] 22, 1031 (1989).
P. Frank, K. A. Bhaskaran, and Th. Just, in Proceedings of the 21st Symposium (International) on Combustion, Pittsburgh, 1988, Symp. (Int.) Combust., [Proc.] 21, 885 (1988).
J. A. Miller, R. E. Mitchell, M. D. Smooke, and R. J. Kee, in Proceedings of the 19th Symposium (International) on Combustion, Pittsburgh, 1982, Symp. (Int.) Combust., [Proc.] 19, 181 (1982).
P. Frank, K. A. Bhaskaran, and Th. Just, J. Phys. Chem. 90, 2226 (1986).
P. Frank and Th. Just, in Proceedings of the 14th International Symposium on Shock Tubes and Waves, Sydney, 1984, Proc. Int. Sump. Shock Tubes Waves, 706 (1984).
Ch. Dombrowsky and H. Gg. Wagner, Ber. Bunsen-Ges. Phys. Chem. 96, 1048 (1992).
A. M. Dean, J. Phys. Chem. 89, 4600 (1985).
S. C. Li and F. A. Williams, in Proceedings of the 26th Symposium (International) on Combustion, Pittsburgh, 1996, Symp. (Int.) Combust., [Proc.] 26, 1017 (1996).
M. Bartels, J. Edelbuttel-Einhaus, and K. Hoyermann, in Proceedings of the 23rd Symposium (International) on Combustion, Pittsburgh, 1990, Symp. (Int.) Combust., [Proc.] 23, 131 (1990).
H. F. Calcotte, in Proceedings of the 8th Symposium (International) on Combustion, Pittsburgh, 1962, Symp. (Int.) Combust., [Proc.] 8, 184 (1962).
J. Peeters and C. Vinckier, in Proceedings of the 15th Symposium (International) on Combustion, Pittsburgh, 1974, Symp. (Int.) Combust., [Proc.] 15, 969 (1974).
B. P. Demidovich, I. A. Maron, and E. Z. Shuvalova, Numerical Analysis Methods (Nauka, Moscow, 1967) [in Russian].
G. A. Askar’yan, G. M. Batanov, A. E. Barkhudarov, S. I. Gritsinin, E. G. Korchagina, I. A. Kossyi, V. P. Silakov, and N. M. Tarasova, Sov. J. Plasma Phys. 18, 625 (1992).
G. M. Batanov, I. A. Kossyi, and V. P. Silakov, Plasma Phys. Rep. 28, 204 (2002).
G. A. Askaryan, G. M. Batanov, I. A. Kossyi, and A. Yu. Kostinskii, in High Power Microwave Generation and Applications (Proceedings of the 10th International School of Plasma Physics “Piero Caldirola,” Varenna, 1991), Ed. by D. K. Akulina, C. B. Wharton, and E. Sindoni (Editrice Compositori, Bologna, 1992), p. 207.
G. A. Askaryan, G. M. Batanov, D. F. Bykov, S. I. Gritsinin, I. A. Kossyi, A. Yu. Kostinskii, A. A. Matveyev, and V. P. Silakov, in Controlled Active Global Experiments (Proceedings of the 7th International School of Plasma Physics “Piero Caldirola,” Varenna, 1990), Ed. by E. Sindoni and A. Y. Wong (Editrice Compositori, Bologna, 1991), p. 239.
V. Shui, J. Chem. Phys. 53, 2547 (1970).
S. W. Benson and T. Fueno, J. Chem. Phys. 36, 1597 (1962).
Funding
This work was supported by the Russian Science Foundation, project no. 17-12-01352.
Author information
Authors and Affiliations
Corresponding author
APPENDIX
APPENDIX
Tables 1–10 show the rate constants and reaction equations used in the model.
Reactions of dissociation and recombination occur with the participation of a third particle (M or Mc). The type of particle M is determined by the chemical compounds listed in Section 2 of this article. The type of particle MC is given in Tables 1–3.
According to literature, the recommended values of reaction rate constants are given taking into account the temperature range T (in K) in the second column of the table. If the temperature range is not given by the authors or is narrower than the temperature range under study (from 300 to 5000 K), then in the model, the rate constants are extrapolated over the temperature range under study.
For most reactions given in the tables, the dependences on temperature of rate constants \({{k}_{{f,r}}}\) of the forward ( f ) and reverse (r) chemical reactions are approximated using the modified Arrhenius equation [28, 32, 33, 35]
Here, the rate constants are expressed in units of mole1 ‒ s cm3(s – 1) s–1. The value s determines the order of the reaction. The parameters A, n, and Ea are the constant preexponential factor, the index of power in the temperature factor of the preexponential factor, and the energy threshold of the reaction, respectively [26, 33].
The rate constants for a number of reactions have a more complicated dependence on the translational temperature than the predictions of the modified Arrhenius formula. Their value can be determined not only by the translational temperature, but also by the concentrations of their interaction partners. These rate macroconstants of complicated reactions are given in tables using the approximate expressions from work [32] obtained by the methods of single-quantum step-wise excitation and transitional state [46], the Troe model, the modified Keck’s variational theory [96], and by the scheme of single-step dissociation [97]. Expressions for such constants of forward and reverse reactions contain correction factors dif and dir, respectively. The lower index i stands for the number of the reaction for which the correction factor is in-troduced. For example, for the forward reaction no. 3.15.7, index i is 3157f.
The correction coefficient \({{d}_{{101f}}}\) for reactions 1.0.1–1.0.5 (Table 1) is calculated by the formula
The coefficients d101r and d102r are determined as the product of the correction coefficient \({{d}_{{{\text{101}}f}}}\) and the equilibrium constant Keq of reactions 1.0.1–1.0.5 (Table 1), respectively.
For reaction 2.0.3 (Table 2), the coefficient d203f changes is the range from 0.3 to 1.5. Coefficients d204 f,r and \({{d}_{{{\text{221}}f}}}\) for reactions 2.0.4 and 2.2.1–2.2.5, 2.3 (Table 2), respectively, are determined by expressions
To determine the values of the correction coefficient \({{d}_{{{\text{301}}f}}}\) (for reactions 3.0.1 and 3.0.2, Table 3) the following relation is used
Coefficients \({{d}_{{{\text{331}}f,r}}}\) for reactions of dissociation and recombination (3.3.1–3.3.5, Table 3) are calculated as
Here, \(K_{{{\text{Ar}}}}^{{\text{0}}}\) is the recombination rate constant (on argon atoms) in the low-pressure region (it is measured in cm6 s–1 mol–2), \({{K}_{\infty }}\) is the recombination rate constant in the high-pressure range (it is measured in cm3 s–1 mol–1), \({{X}_{m}}\) are the molar fractions of the chemical compounds of the mixture (m = \({{{\text{N}}}_{{\text{2}}}}{\text{,}}\;{{{\text{O}}}_{{\text{2}}}}{\text{,}}\;{\text{NO,}}\;{{{\text{N}}}_{{\text{2}}}}{\text{O,}}\;{\text{N}}{{{\text{O}}}_{{\text{2}}}}\)), NA is the number of particles in a single mole of the substance, and \({{R}_{m}}\) is the relative efficiency of component m relative to component m = \({{{\text{N}}}_{{\text{2}}}}\):
The values of coefficients \({{d}_{{{\text{371}}f,r}}}\) (for reactions 3.7.1, Table 3) and \({{d}_{{{\text{311}}f}}}\) (for reaction 3.11, Table 3) are calculated by the formulas
For reaction 3.11, index m, besides the listed components for the mixture for reactions 3.7.1., additionally includes the denomination for the CO2 component (\(m = {\text{C}}{{{\text{O}}}_{{\text{2}}}}\)).
The coefficients \({{d}_{{{\text{3151}}f,r}}}\) and \({{d}_{{{\text{3154}}f,r}}}\) for reactions of dissociation and recombination (3.15.1 and 3.15.4, Table 3) are determined by the expressions
Here, \(K_{{{{{\text{N}}}_{{\text{2}}}},f}}^{{\text{0}}}\) and \(K_{{{{{\text{N}}}_{{\text{2}}}},r}}^{{\text{0}}}\) are the rate constants for the reactions of dissociation and recombination in the low-pressure range (their units of measurement are cm3 s–1 mol–1 and cm6 s–1 mol–2, respectively). \(K_{{\infty ,f}}^{{}}\) and \(K_{{\infty ,r}}^{{}}\) are the rate constants in the high-pressure region (units of measurement s–1 and cm3 s–1 mol–1, respectively). The indices 1 and 4 correspond to molecular components \({{{\text{N}}}_{{\text{2}}}}{\text{,}}{{{\text{O}}}_{{\text{2}}}}\) and \({\text{CO,C}}{{{\text{O}}}_{{\text{2}}}}\).
The coefficients \({{d}_{{{\text{3161}}f,r}}}\) and \({{d}_{{{\text{3162}}f,r}}}\) for reactions of dissociation and recombination (3.16.1 and 3.16.2, Table 3) are found by the expressions
Here, indices 1 and 2 correspond to the \({{{\text{N}}}_{{\text{2}}}}\), O2 and \({\text{CO,}}\;{\text{C}}{{{\text{O}}}_{{\text{2}}}}\) components of the gas medium.
The coefficients \({{d}_{{{\text{3171}}f,r}}}\) of dissociation and recombination reactions (3.17.1, Table 3) are calculated by the formulas
The coefficient d323f for reaction 3.23 (Table 3) is the same as coefficient d331f (dissociation reaction 3.3.1–3.3.5, Table 3). For this reaction, \({{X}_{m}}\) are mole fractions of the mixture components m = \({\text{N}}{{{\text{O}}}_{{\text{2}}}}{\text{,}}{{{\text{N}}}_{{\text{2}}}}{\text{,}}{{{\text{O}}}_{{\text{2}}}}{\text{,C}}{{{\text{O}}}_{{\text{2}}}}\) and Rm is the relative efficiency of component m relative to the component m = \({\text{N}}{{{\text{O}}}_{{\text{2}}}}\):
The values of coefficients \({{d}_{{{\text{3271}}f,r}}}\) for dissociation and recombination reactions (3.27.1, Table 3) are determined by the expressions
To determine the rate constants for reactions 1.0.1–1.0.5 (Table 1), 2.2.1–2.2.5 (Table 2), and 3.22 (Table 3), the detailed equilibrium principle is applied. They are calculated taking into account the equilibrium constant Keq. The tabulated data for the equilibrium constants are taken from reference book [19]. The dependence of the equilibrium constant on temperature is interpolated by the method of cubic splines.
For the comparative analysis of the rate constants of reactions reverse to dissociation (recombination reactions from Tables 1–3), they were also determined by the model of triple collisions [33]. The collision cross sections of the particles are calculated by the models of elastic collisions: hard spheres, hard spheres with variable diameter; with Lennard-Jones interaction potential; with Born–Mayer repulsion potential.
Rights and permissions
About this article
Cite this article
Shakhatov, V.A., Gritsinin, S.I. & Borzosekov, V.D. Simulations of the Formation of Nitrogen Oxides at the Cooling Stage of a Subthreshold Microwave Discharge in Air with Admixtures of Methane. Plasma Phys. Rep. 47, 465–497 (2021). https://doi.org/10.1134/S1063780X21050081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063780X21050081