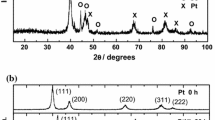
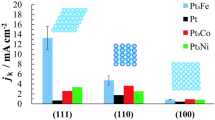
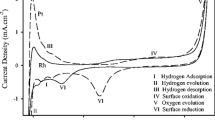
Abstract
The characteristics of oxygen reduction on various platinum electrodes (smooth polycrystalline metal, Pt(hkl) single-crystals, disperse materials) in alkaline media are interpreted. The interpretation was based on the theory of electrochemical processes with a slow subsequent heterogeneous reaction. The dependence of polarization curve slopes in Tafel coordinates on the type of electrode material surface is explained based on the Temkin approach as the energetic inhomogeneity of the electrode surface. The energetic inhomogeneity is associated with the appearance of surface hydroxide nanoclusters of various composition based on oxidized platinum atoms which simultaneously represent the surface lattice defects s, dPt. The multistage heterogeneous chemical reaction is interpreted as the innercluster oxidation of the previously electrochemically reduced s, dPt atoms by oxygen molecules.
Similar content being viewed by others
References
Appleby, A.J., J. Electroanal. Chem., 1993, vol. 357, pp. 117.
Tarasevich, M.R. and Khrushcheva, E.I., Kinetika slozhnykh elektrokhimicheskikh reaktsii (Kinetics of Complex Electrochemical Reactions), Moscow: Nauka, 1981.
Tarasevich, M.R, Sadkowski, A., and Yeager, E., in Comprehensive Treatise of Electrochemistry, vol. 7, Conway, B.E., Bockris, J.O’M., Yeager, E., Kahn, U.M.S., and White, R.E., Eds., New York: Plenum Press, 1983, pp. 301–398.
Damjanovic, A., in Modern Aspect of Electrochemistry, vol. 5, Bockris, J.O’M. and Conway, B.E., Eds., New York: Plenum Press, 1969, pp. 369–466.
Sugawara, S., Tsujita, K., Mitsushima, S., Shinohara, K., and Ota, K., Electrocatal., 2011, vol. 2, p. 40.
Obradovic, M.D., Grgur, B.N., and Vragar, Lj.M., J. Electroanal. Chem., 2003, vol. 548, p. 69.
Anderson, A.B., Electrocatalysis, 2012, vol. 3, p. 176.
Bockris, J.O’M. and Abdu, R., J. Electroanal. Chem., 1998, vol. 448, p. 189.
Rotinyan, A.L., Tikhonov, K.I., and Shoshina, I.A., Teoreticheskaya elektrokhimiya (Theoretical Electrochemistry, Leningrad: Khimiya, 1981.
Sepa, D., Vojnovic, M., and Damjanovic, A., Electrochim. Acta, 1981, vol. 26, p. 781.
Lima, F.H.B., Calegaro, M.L., and Ticianelli, E.A., Russ. J. Electrochem., 2006, vol. 42, p. 1283.
Haijing Liu, Jin Li, Xinhua Xu, Feng Wang, Jingjun Liu, Zhilin Li, and Jing Ji, Electrochim. Acta, 2013, vol. 93, p. 25.
Sepa, D.B., Vojnovic, M.V., and Vracar, Lj.M., Electrochim. Acta, 1984, vol. 29, p. 1169.
Sepa, D.B., Vojnovic, M.V., Vracar, Lj.M., and Damjanovic, A., Electrochim. Acta, 1986, vol. 31, p. 97.
Rao, M.L.B., Damjanovic, A., and Bockris, J.O’M., J. Phys. Chem., 1963, vol. 67, p. 2508.
Trunov, A.M., Electrokhimiya, 1986, vol. 22, p. 1093.
Trunov, A., Electrochim. Acta, 2013, vol. 105, p. 506.
Schmidt, T.J., Stamenkovic, V., Ross, P.N., and Jr.Markovic, N.M., Phys. Chem. Chem. Phys., 2003, vol. 5, p. 400.
Schmidt, T.J., Stamenkovic, V., Arenz, M., Markovic, N.M., and Ross, P.N., Jr., Electrochim. Acta, 2002, vol. 47, p. 3765.
Deryagina, O.G, Paleolog, E.N., Electrokhimiya, 1972, V. 8, p. 431 (in Russian).
Damjanovic, A., Dey, A., and Bockris, J.O’M., Electrochim. Acta, 1966, vol. 11, p. 791.
Sepa, D.B., Vojnovic, M.V., Vracar, Lj.M., and Damjanovic, A., Electrochim. Acta, 1986, vol. 31, p. 91.
Wroblowa, H., Rao, M.L.B., Damjanovic, A., and Bockris, J.O’M., J. Electroanal. Chem., 1967, vol. 15, p. 139.
Damjanovic, A. and Brusic, V., Electrochim. Acta, 1967, vol. 12, p. 615.
Bagotskii, V.S. and Tarasevich, M.R., J. Electroanal. Chem., 1979, vol. 101, p. 1.
Conway, B.E., Prog. Surf. Sci., 1995, vol. 49, p. 331.
Angerstein-Kozlovska, H., Conway, B.E., and Hamelin, A., Electrochim. Acta, 1986, vol. 31, p. 1051.
Energiya razryva khimicheskikh svyazei. Potenstialy ionizatsii i srodstvo k elektronu (Breakup Energy of Chemical Bonds. Potentials of Ionization and Electron Affinity), Moscow: AN SSSR, 1962.
Spravochnik po elektrokhimii (Electrochemistry Handbook, Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Yeager, E., Electrochim. Acta, 1984., vol. 29, p. 1527.
Yeager, E., J. Mol. Catal., 1986, vol. 38, p. 5.
Trunov, A.M., Abstracts of Papers, V Konferentsiya “Sovremennye metody v teoreticheskoi i eksperimental’noi elektrokhimii” (V Conference “Contemporary Methods of Theoretical and Experimental Electrochemistry”), Ivanovo, 2013, p. 180.
Trunov, A.M., Abstracts of Papers, Vserossiiskaya konferentsiya “Fiziko-khimicheskie problemy vozobnovlyaemoi energetiki” (All-Russian Conference “Physicochemical Problems of Renewable Energetics”), St.-Petersburg: Polytechnical University, 2013, p. 144.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.M. Trunov, 2015, published in Elektrokhimiya, 2015, Vol. 51, No. 4, pp. 385–392.
Rights and permissions
About this article
Cite this article
Trunov, A.M. On the mechanism of oxygen reduction on single-crystal and polycrystalline Pt electrodes in alkaline media. Russ J Electrochem 51, 332–338 (2015). https://doi.org/10.1134/S1023193515040138
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193515040138