Abstract
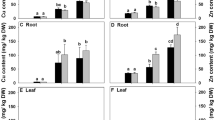
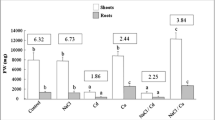
Seedlings of groundnut (Arachis hypogaea L.) ‘TG-51’ were raised in sand culture in presence of three different concentrations of cadmium (100, 300 and 500 µM) and two concentrations of zinc (50 and 150 µM) along with their combinations. In general, the root accumulated several times higher content of both the metals than leaf. It was noted that uptake of Cd by root decreased in combined treatment in comparison with the sole application of cadmium. The dry weight of root, stem, leaf as well as the whole seedling significantly decreased under cadmium stress compared to unstressed control. Zinc supplementation in the growing medium minimized the adverse effects of cadmium on seedling growth to some extent but could not completely override it. Presence of zinc in combination with cadmium could further the enhancement of proline accumulation under heavy metal stress at 100 and 300 µM Cd but not at the highest concentration. Zinc supplementation in combination with cadmium also lowered the damaging effect of cadmium on leaf membrane to some extent.Higher supplement of Zn was able to enhance dismutation of Cd-triggered superoxide radical by superoxide dismutase enzyme, facilitating its subsequent detoxification through the involvement of peroxidase and catalase enzymes, thereby highlighting its protective role against oxidative damage under heavy metal stress. The results of PCA indicated that strong positive loadings of dry weight of root, leaves as well as whole seedling at PC 1 (55.7%) while the activities of SOD, GPOX and catalase enzymes at PC 2 (16%) mostly contributed towards discrimination among different treatments.



Similar content being viewed by others
REFERENCES
Seregin, I.V. and Ivanov, V.B., Physiological aspects of cadmium and lead toxic effects on higher plants, Russ. J. Plant Physiol., 2001, vol. 48, p. 523.
Khan, N.A., Singh, S., and Nazar, R., Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress, J. Agron. Crop Sci., 2007, vol. 193, p. 435.
Adil, M.F., Sehar, S., Han, Z., Lwalaba, J.L.W., Jilani, G., Zeng, F., Chen, Z.H., and Shamsi, I.H., Zinc alleviates cadmium toxicity by modulating photosynthesis, ROS homeostasis, and cation flux kinetics in rice, Environ. Pollut., 2020, vol. 265, p. 114979.
Broadley, M., Brown, P., Cakmak, I., Rengel, Z., and Zhao, F., Function of nutrients: micronutrients, in Marschner’s Mineral Nutrition of Higher Plants, London: Academic, 2012.
Parlak, U.K. and Yilmaz, D.D., Response of antioxidant defences to Zn stress in three duckweed species, Ecotoxicol. Environ. Saf., 2012, vol. 85, p. 52.
Narwal, R.P., Singh, M., Singh, J.P., and Dahiya, D.J., Cadmium-zinc interaction in maize grown on sewer water irrigated soil, Arid Soil Res. Rehabil., 1993, vol. 7, p. 125.
Cherif, J., Mediouni, C., Ammar, W.B., and Jemal, F., Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solanum lycopersicum), J. Environ. Sci., 2011, vol. 23, p. 837.
Hart, J.J., Welch, R.M., Norvell, W.A., and Kochian, L.V., Transport interactions between cadmium and zinc in root of bread and durum wheat seedlings, Physiol. Plant., 2002, vol. 116, p. 73.
Aravind, P. and Prasad, M.N.V., Cadmium-zinc interaction in a hydroponic system using Ceratophyllum demersum L.: adaptive ecophysiology, biochemistry and molecular toxicology, Braz. J. Plant Physiol., 2005, vol. 17, p. 3.
McLaughlin, M.J., Bell, M.J., Wright, G.C., and Cozens, G.D., Uptake and partitioning of cadmium by cultivars of peanut (Arachis hypogaea L.), Plant Soil, 2000, vol. 222, p. 51.
Dinakar, N., Nagajyothi, P.C., Suresh, S., Damodharam, T., and Suresh, C., Cadmium induced changes on proline, antioxidant enzymes, nitrate and nitrite reductases in Arachis hypogaea L., J. Environ. Biol., 2009, vol. 30, p. 289.
Epstein, E., Mineral Nutrition of Plants: Principles and Perspectives, New York: Wiley, 1972.
Jackson, M.L., Soil Chemical Analysis, Englewood Cliffs, NJ: Prentice Hall, 1962.
Arnon, D.I., Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris, Plant Physiol., 1949, vol. 24, p. 1.
Heath, R.L. and Packer, L., Photoperoxidaton in isolated chloroplast. 1. Kinetics and stoichiometry of fatty acid peroxidation, Arch. Biochem. Biophys., 1968, vol. 12, p. 189.
Guo, Z., Ou, W., Lu, S., and Zhong, Q., Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity, Plant Physiol. Biochem., 2006, vol. 44, p. 828.
Mohanty, S.K. and Sridhar, R., Physiology of rice tungro virus disease: proline accumulations due to infection, Physiol. Plant., 1982, vol. 56, p. 89.
Jaworski, E.G., Nitrate reductase assay in intact plant tissues, Biochem. Biophys. Res. Commun., 1971, vol. 43, p. 1274.
Giannopolitis, C.N. and Ries, S.K., Superoxide dismutase: I. Occurrence in higher plants, Plant Physiol., 1977, vol. 59, p. 309.
Goth, L., A simple method for determination of serum catalase activity and revision of reference range, Clin. Chim. Acta, 1991, vol. 196, p. 143.
Siegel, B.Z. and Galston, A.W., The isoperoxidases of Pisum sativum, Physiol. Plant., 1967, vol. 42, p. 212.
Burzynski, M. and Zurek, A., Effects of copper and cadmium on photosynthesis in cucumber cotyledons, Photosynthetica, 2007, vol. 45, p. 239.
Brown, P.H., Cakmak, I., and Zhang, Q., Form and function of zinc plants, in Zinc in Soils and Plants, Dordrecht: Springer-Verlag, 1993, p. 93.
Sagardoy, R.S., Vázquez, I.D., Florez-Sarasa, A., Albacete, M., Ribas-Carbó, J., Flexas, J., Abadía, J., and Morales, F., Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc, New Phytol., 2010, vol. 187, p. 145.
Rascio, N., Dalla Vecchia, F., La Rocca, N., Barbato, R., Pagliano, C., Raviolo, M., Gonnelli, C., and Gabbrielli, R., Metal accumulation and damage in rice (cv. Vialonenano) seedlings exposed to cadmium, Environ. Exp. Bot., 2008, vol. 62, p. 267.
Symeonidis, L. and Karataglis, S., Interactive effects of cadmium, lead and zinc on root growth of two metal tolerant genotypes of Holcuslanatus L., Biometals, 1992, vol. 5, p. 173.
Myśliwa-Kurdziel, B., Prasad, M.N.V., and Strzałka, K., Photosynthesis in heavy metal stressed plants, in Heavy Metal Stress in Plants: From Biomolecules to Ecosystems, Berlin: Springer-Verlag, 2004, ch. 6.
Kawano, T., Kawano, N., Muto, S., and Lapeyrie, F., Cation-induced superoxide generation in tobacco cell suspension culture is dependent on ion valence, Plant Cell Environ., 2001, vol. 24, p. 1235.
Powell, S.R., The antioxidant properties of zinc, J. Nutr., 2000, vol. 130, p. 1447.
Luna, C.M., González, V.S., and Trippi, V.S., Oxidative damage caused by excess copper in oat leaves, Plant Cell Physiol., 1994, vol. 35, p. 11.
ACKNOWLEDGMENTS
The authors acknowledge the assistance extended by AICRP on Groundnut, Kalyani centre, Bidhan Chandra Krishi Viswavidyalaya for supplying plant materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Abbreviations: CAT—Catalase, EL—electrolyte leakage, GPOX—guaiacol peroxidase, NR—nitrate reductase, PCA— principal component analysis, SOD—superoxide dismutase, TBARS—thiobarbituric acid reactive substances.
Rights and permissions
About this article
Cite this article
Dutta, D., Pal, A.K. & Acharjee, P.U. Physiological Studies on Seedling Growth in Groundnut (Arachis hypogaea L.) under Interactive Effects of Cadmium and Zinc. Russ J Plant Physiol 68 (Suppl 1), S82–S91 (2021). https://doi.org/10.1134/S1021443721070025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721070025