Abstract
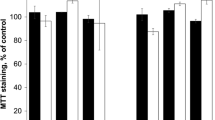
Amphiphilic copolymers of N-vinylpyrrolidone and methacrylic acid, and triethylene glycol dimethacrylate (branching agent) are synthesized by radical copolymerization in toluene and ethanol; their main physicochemical characteristics (monomer composition, absolute weight-average molecular weight, polydispersity, and hydrodynamic radius in water and aqueous media with various рН) are determined. It is shown that in weakly acidic media copolymer solutions exhibit the lower critical temperature which shifts to high values in neutral and alkaline aqueous buffered solutions. Water-soluble nanoscale systems of red-fluorescent dye zinc tetraphenylporphyrinate having a hydrodynamic radius of about 50 nm (in aqueous neutral buffered solution) and stable in the physiologically important temperature range are obtained. Using the МТТ assay on various cell lines, normal (FetMSC, Vero) and tumor (HepG2, HeLa), ternary copolymers are found to be low toxic. The dynamics of accumulation of dye-loaded polymer particles in HeLa and Vero cells is studied by fluorescence microscopy. It is concluded that the synthesized copolymers show promise as carriers and means of delivery of biologically active compounds.









Similar content being viewed by others
REFERENCES
Yu. E. Kirsh, Poly(N-vinylpyrrolidone) and Other Poly(N-vinylamides) (Nauka, Moscow, 1998).
E. F. Panarin, N. A. Lavrov, M. V. Solovskii, and L. I. Shal’nova, Polymers as Carriers of Biologically Active Compounds (Professiya, St. Petersburg, 2014).
F. P. Sidel’kovskaya, Chemistry of N-Vinylpyrrolidone and Its Polymers (Nauka, Moscow, 1970).
H. Folttmann and A. Quadir, Drug Deliv. Technol. 8, 22 (2008).
C. Bothiraja, M. B. Shinde, S. Rajalakshmi, and A. P. Pawar, J. Pharm. Pharmacol. 61, 1465 (2009).
R. M. Martins, S. V. Pereira, S. Siqueira, W. F. Salomão, and L. A. P. Freitas, Food Res. Int. 50, 657 (2013).
M. Rasekh, C. Karavasili, Y. L. Soong, N. Bouropoulos, M. Morris, D. Armitage, X. Li, D. G. Fatouros, and Z. Ahmad, Int. J. Pharm. 473, 95 (2014).
R. Fogaça and L. H. Catalani, Soft Mater. 11, 61 (2013).
I. D. Del Consuelo, F. Falson, R. H. Guy, and Y. Jacques, J. Control. Release 122, 135 (2007).
Paola Franco and Iolanda De Marco, Polymers 12, 1114 (2020).
X. Zheng, T. Zhang, X. Song, L. Zhang, C. Zhang, S. Jin, J. Xing, and X.-J. Liang, J. Mater. Chem. B 3, 4027 (2015).
L. Zhang, Y. Liang, L. Meng, and C. Wang, Polym. Adv. Technol. 20, 410 (2009).
Y. Song, T. Zhang, X. Song, L. Zhang, C. Zhang, J. Xing, and X.-J. Liang, J. Mater. Chem. B 3, 911 (2015).
A. Saxena, S. Mozumdar, and A. K. Johri, Biomaterials 27, 5596 (2006).
S.-J. Sheu, L.-C. Chou, Y.-S. Bee, J.-F. Chen, H.-C. Lin, P.-R. Lin, H.-C. Lam, and M.-H. Tai, Exp. Eye Res. 81, 673 (2005).
V. Ramalingam, K. Varunkumar, V. Ravikumar, and R. Rajaram, Sci. Rep. 8, 1 (2018).
P. A. Rose, P. Praseetha, M. Bhagat, P. Alexander, S. Abdeen, and M. Chavali, Technol. Cancer Res. Treat. 12, 463 (2013).
M. Hu, C. Li, X. Li, M. Zhou, J. Sun, F. Sheng, S. Shi, and L. Lu, Artif. Cells Nanomed. Biotechnol. 46, 1248 (2018).
M. Hecold, R. Buczkowska, A. Mucha, J. Grzesiak, O. Rac-Rumijowska, H. Teterycz, and K. Marycz, J. Nanomater. 2017, 8706921 (2017).
G. de Lima, D. W. de Lima, M. J. de Oliveira, A. B. Lugão, M. S. Alcántara, D. M. Devine, and M. J. de Sá, ACS Appl. Bio Mater. 1, 1842 (2018).
H. Besrour, B. Tangour, R. Linguerri, and M. Hochlaf, Spectrochim. Acta A 217, 278 (2019).
X. Zeng, Y. Zhang, Z. Wu, P. Lundberg, M. Malkoch, and A. M. Nyström, J. Polym. Sci., Part A: Polym. Chem. 50, 280 (2011).
Y. Zhou and D. Yan, Chem. Commun. 9, 1172 (2009).
Y. Zhou, W. Huang, J. Liu, X. Zhu, and D. Yan, Adv. Mater. 22, 4567 (2010).
M. V. Solovskij, N. V. Nikolskaya, E. B. Tarabukina, V. M. Denisov, A. V. Adamov, and S. I. Klenin, Des. Monomers Polym. 2, 83 (2004).
S. V. Kurmaz and A. N. Pyryaev, Polym. Sci., Ser. B 52 (1), 1 (2010).
S. V. Kurmaz, N. V. Fadeeva, Y. V. Soldatova, I. I. Faingold, D. A. Poletaeva, V. M. Ignat’ev, N. S. Emel’yanova, G. V. Shilov, and R. A. Kotelnikova, J. Polym. Res. 28, 345 (2021).
S. V. Kurmaz, V. D. Sen’, A. V. Kulikov, D. V. Konev, V. A. Kurmaz, A. A. Balakina and A. A. Terent’ev, Russ. Chem. Bull. 68, 1769 (2019).
S. V. Kurmaz, N. V. Fadeeva, B. S. Fedorov, G. I. Kozub, N. S. Emel’yanova, V. A. Kurmaz, R. A. Manzhos, A. A. Balakina, and A. A. Terentyev, Mendeleev Commun. 30, 22 (2020).
S. V. Kurmaz, N. V. Fadeeva, B. S. Fedorov, G. I. Kozub, V. A. Kurmaz, V. M. Ignat’ev, and N. S. Emel’yanova, Russ. Chem. Bull. 70, 1832 (2021).
S. V. Kurmaz, N. A. Obraztsova, A. A. Balakina, and A. A. Terent’ev, Russ. Chem. Bull. 65, 2097 (2016).
S. V. Kurmaz, N. V. Fadeeva, A. V. Komendant, V. M. Ignatiev, N. S. Emelyanova, G. V. Shilov, T. S. Stupina, N. V. Filatova, M. A. Lapshina, and A. A. Terentyev, Polym. Bull. 10 (2021).
A. N. Zelikin, A. D. Price, and B. Stadler, Small 6, 2201 (2010).
O. V. Zhukova, E. V. Arkhipova, T. F. Kovaleva, S. A. Ryabov, I. P. Ivanova, A. A. Golovacheva, D. A. Zykova, and S. D. Zaitsev, Molecules 26, 4855 (2021).
J. C. Leyte and M. J. Mandel, J. Polym. Sci., Part A: Polym. Chem. 2, 1879 (1964).
S. Graham, P. A. G. Cormack, and D. C. Sherrington, Macromolecules 38, 86 (2005).
N. O’Brien, A. McKee, D. C. Sherrington, A. T. Slark, and A. Titterton, Polymer 41, 6027 (2000).
G. Bianco and H. H. Gehlen, J. Photochem. Photobiol., A 149, 115 (2002).
G. Gergiev and I. Dakova, Colloid Polym. Sci. 272, 938 (1994).
S. V. Kurmaz, N. V. Fadeeva, V. M. Ignat’ev, V. A. Kurmaz, S. A. Kurochkin, and N. S. Emel’yanova, Molecules 25, 6015 (2020).
S. Irvin-Choy N’Dea, K. M. Nelson, J. P. Gleghorn, and E. S. Day, J. Mater. Chem. B 8, 6548 (2020).
E. Blanco, H. Shen, and M. Ferrari, Nat. Biotechnol. 33, 941 (2015).
N. Hoshyar, S. Gray, H. Han, and G. Bao, Nanomedicine 11, 673 (2016).
Funding
This work was carried out in terms of State Assignments АААА-А19-119041090087-4 and АААА-А19-119071890015-6 using equipment of the Analytical Shared Research Center, Institute of Problems of Chemical Physics, Russian Academy of Sciences (https://equipments.icp.ac.ru/ru/equipments/ckp/ackp).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Soboleva
Supplementary Information
Rights and permissions
About this article
Cite this article
Kurmaz, S.V., Ivanova, I.I., Fadeeva, N.V. et al. New Amphiphilic Branched Copolymers of N-Vinylpyrrolidone with Methacrylic Acid for Biomedical Applications. Polym. Sci. Ser. A 64, 434–446 (2022). https://doi.org/10.1134/S0965545X22700237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X22700237