Abstract
New iron(II) coordination compounds with 2,6-bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine (L) and closo-borate(2–) anions [FeL2]B10H10⋅2H2O and [FeL2]B12H12⋅H2O have been synthesized. The compounds have been identified and studied by CHN analysis, electron spectroscopy (diffuse reflection spectroscopy), IR, Mössbauer, and EXAFS spectroscopies, X-ray powder diffraction, and static magnetic susceptibility. The structures of the coordination knots of complexes [FeL2]B10H10⋅2H2O and [FeL2]B12H12⋅H2O has been obtained by modeling the EXAFS spectra. The ligand is coordinated by the iron(II) ion in a tridentate-cyclic manner by two nitrogen atoms of imidazole cycles and a nitrogen atom of pyridine to form the FeN6 coordination knot. The study of the temperature dependence of the magnetic susceptibility in the range of 80–500 K has showed that the high-temperature spin-crossover 1А1 ↔ 5Т2 manifests itself in the obtained compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The spin crossover phenomenon invariably attracts the attention of researchers and is intensively studied [1–9]. Spin crossover is observed in metal complexes with the 3d4–3d7 electronic configuration predominantly in the octahedral or pseudooctahedral environment of the ligands. Under the influence of external conditions (temperature, pressure, irradiation with light of a certain wavelength, and other factors), the spin multiplicity of the central atom can change. Compounds with spin crossover have the ability to exist in two states with a sufficiently long lifetime: low-spin (LS) and high-spin (HS). This serves as a prerequisite for their use as the element base of electronic devices [3, 10–15]. Iron(II) complexes with polynitrogen-containing heterocyclic ligands are a promising class of compounds with such properties. At a certain strength of the ligand field, the 1А1 ↔ 5Т2 spin crossover is observed in them. The Novosibirsk group previously synthesized and studied representative series of iron(II) complexes with two classes of polynitrogen ligands: 1,2,4-triazoles [16–19] and tris(pyrazol-1-yl)methanes [4, 9]. A series of iron(II) compounds with 1,2,4-triazole and its 4-substituted derivatives and various outer-sphere anions has been obtained. It should be noted that the interest in 1,2,4-triazoles as ligands among researchers involved in spin crossover has not weakened over a number of years. Recently, two papers have been published that report a new, rather unexpected application of the complexes synthesized in our group [20, 21]. The study [20] describes a new approach to the development of 4D printing based on the use of nanocomposites with spin crossover. 4D printers can be manufactured by using smart, responsive materials to change the shape and/or function of a 3D printed object. To distinguish these objects from static 3D printed structures, the term 4D printing has been used, where the fourth dimension refers to time. To achieve this goal, the authors chose and successfully applied the previously synthesized iron(II) complex with 4-amino-1,2,4-triazole of the composition Fe(NH2trz)3SO4 [18] as a complex with spin crossover. The work [21] is devoted to the search for the dependence of the sensitivity of explosives with spin crossover on the state of the central atom. The authors studied the complex of iron(II) perchlorate with unsubstituted 1,2,4-triazole of the composition Fe(Htrz)3(ClO4)2 [16, 19], which we obtained earlier, in which spin crossover is manifested. Investigation of the relationship between the characteristics of the spin-crossover and the sensitivity of the complex to external influences, such as impact, friction, etc. showed that there is a correlation between the LS- or HS-form of the complex and its sensitivity. It was found that Fe(Htrz)3(ClO4)2 in the LS form has a lower impact sensitivity compared to the HS form of this complex. The authors expect that compounds with spin crossover and sensitivity to external influences will become a new class of switchable explosives.
At present, we are engaged in the synthesis and study of Fe(II) complexes with 2,6-bis(imidazol-2-yl)pyridines. Fe(II) complexes with 2,6-bis(benzimidazol-2-yl)pyridine and various outer-sphere anions were prepared and studied [22–26], which exhibit spin crossover. Along with ligands of this class, spin crossover is studied in iron(II) complexes with 2,6-bis(pyrazol-1-yl)pyridine and its derivatives [27–29]. In continuation of our studies, we obtained complexes of a number of iron(II) salts with a new ligand that we synthesized, 2,6-bis(4,5-dimethyl-1Н-imidazol-2-yl)pyridine (L) [30], in which the 1A1 ↔ 5T2 spin crossover is observed. In this work, we synthesized and studied iron(II) complexes with L and closo-borate(2–) ions [FeL2]B10H10⋅2H2O and [FeL2]B12H12⋅H2O.
Compounds containing boron cluster anions are promising for the development of cytotoxic drugs and drugs for neutron capture therapy of tumors [31–35]. The study of the previously obtained iron(II) complexes with a number of polynitrogen ligands containing \({{{\text{B}}}_{{10}}}{\text{H}}_{{10}}^{{2 - }}\) and \({{{\text{B}}}_{{12}}}{\text{H}}_{{12}}^{{2 - }}\) as outer-sphere anions [26, 36, 37] showed that they exhibit a spin crossover. It seemed appropriate to continue research in this direction.

2,6-Bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine (L)
EXPERIMENTAL
Chemicals FeSO4⋅7H2O (AcrosOrganics), ascorbic acid (medical), and rectified ethanol were used for the synthesis. K2B10H10⋅2H2O and K2B12H12 were obtained according to the procedure [38]. All reagents were used without further purification. 2,6-Bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine (L) was synthesized according to the procedure reported [30], its spectral characteristics corresponded to those given in the literature.
Synthesis of [FeL2]B10H10⋅2H2O (1·2H2O), [FeL2]B12H12⋅H2O (2·H2O). A portion of 0.07 g (0.25 mmol) of FeSO4⋅7H2O salt was dissolved in 6 mL of distilled water acidified with 0.05 g of ascorbic acid. closo-Borate salts were added to the resulting solution under stirring: 0.09 g (0.4 mmol) K2B10H10⋅2H2O or 0.09 g (0.4 mmol) K2B12H12 in 5 mL of water. Then, a heated solution of 0.15 g (0.54 mmol) of L in 5 mL of ethanol was slowly added to each of the resulting solutions with stirring. Immediately after mixing the solutions, burgundy precipitates formed, which were stirred on a magnetic stirrer for 5 h. The precipitates were filtered off, washed twice with 1 mL of water and 1 mL of ethanol, and dried in air. The yield of compounds 1 and 2 was 93 and 95%, respectively.
For C30H48B10FeN10O2, anal. calcd. (%): C, 48.4; H, 6.5; N, 18.8.
Found (%): C, 48.9; H, 6.3; N, 18.4.
For C30H50B12FeN10O2, anal. calcd. (%): C, 46.9; H, 6.6; N, 18.2.
Found (%): C, 46.9; H, 6.5; N, 17.8.
Elemental analysis of the ligand and complexes for C, H, N was performed on a Euro EA 3000 instrument from EuroVector (Italy).
X-ray powder diffraction studies of polycrystalline compounds were performed on a Shimadzu XRD 7000 diffractometer (CuKα radiation, Ni filter, scintillation detector) at room temperature.
X-ray absorption spectra in the region of the Fe K-edge (150–800 eV above the absorption edge) were obtained on channel 8 of the storage ring VEPP-3 of the Siberian Center for Synchrotron and Terahertz Radiation of the Budker Institute of Nuclear Physics, Syberian Branch, Russian Academy of Sciences [39]. The spectra were obtained in the standard transmission mode using ionization chambers filled with Ar/He (monitoring detector) and Xe (final detector) gas mixtures. A slotted Si(111) single crystal was used as the input crystal of the monochromator. The energy of the storage ring during measurements was 2 GeV at a current value of 70–140 mA. For measurements, the samples were mixed with powdered cellulose as a filler and pressed into tablets. The amount of sample was calculated to provide the absorption jump to be Δμx = 0.8–1.0 at the Fe K-edge. To measure the absorption spectra at an elevated temperature for the complexes in the HS state, the samples were placed in a tube furnace open at the ends. The temperature in the middle of the oven, where the sample was located, was maintained at a level of 420 ± 5 K using a THERMODAT 10K thermostat.
IR spectra of the complexes were recorded on a Scimitar FTS 2000 IR Fourier spectrometer in the 4000–400 cm–1 region and a Vertex 80 400–100 cm–1 region. Samples were prepared as suspensions in Vaseline and fluorinated oil and polyethylene. The IR spectra of the ligand were recorded in KBr pellets on a Bruker Vector-22 spectrometer, the UV-visible spectrum of the ligand was obtained using a Hewlett-Packard HP 8453 spectrophotometer.
1Н and 13С NMR spectra of the ligand were recorded on a Bruker AV-300 spectrometer at 300 and 75 MHz, respectively. Chemical shifts were determined relative to the signals of the residual solvent (DMSO-d6: 2.50 ppm for 1Н nuclei and 39.5 ppm for 13С nuclei), melting temperature, on a Mettler Toledo FP-900 instrument.
Diffuse reflectance Kubelka–Munk spectra were recorded on a Shimadzu UV-3101 RS scanning spectrophotometer at room temperature.
Static magnetic susceptibility of the samples was measured by the Faraday method in the temperature range of 80–520 K. The temperature stabilization of the sample with an accuracy of 1 K during the measurement was carried out using a PID controller DTB9696 from Delta Electronics. The rate of heating and cooling of the samples was ~2–3 deg/min. An external magnetic field strength of 7.3 kOe was maintained during the studies with a stabilization accuracy of ~2%. To study the synthesized compounds containing water of crystallization, the samples were sealed with atmospheric air into quartz ampoules. To study the dehydrated complexes, the starting compounds were placed in open quartz ampoules and evacuated to a residual pressure of 10–2 mmHg in the measuring cell of the setup, then created an inert atmosphere of helium at a pressure of 5 mm Hg. The temperatures of the forward (Тс↑) and reverse (Тс↓) transitions were determined based on the condition d2µeff/dT2 = 0. The effective magnetic moment was calculated using the formula µeff = \({{\left( {8\chi _{{\text{M}}}^{'}T} \right)}^{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0em} 2}}}},\) where \(\chi _{{\text{M}}}^{'}\) is the molar magnetic susceptibility corrected for the diamagnetism of atoms according to the Pascal scheme.
Mössbauer spectra of the complexes were measured at room temperature on an NP-610 spectrometer with a 57Co (Rh) source. As a result of processing the spectra, the isomeric shift δ (with respect to α-Fe) and the quadrupole splitting ε were found.
RESULTS AND DISCUSSION
Compounds [FeL2]B10H10⋅2H2O and [FeL2]B12H12⋅H2O were obtained from aqueous ethanol solutions at an iron(II) salt concentration of ~0.1 mol/L and a Fe : L stoichiometric ratio. Ascorbic acid was added as a reducing agent and weakly acidifying agent to an iron(II) solution. The synthesis was carried out in two stages. At the first stage, a solution of iron(II) closo-borates was obtained from an aqueous solution of FeSO4 using a 1.5-fold excess of salts K2B10H10⋅2H2O or K2B12H12. At the second stage, a solution of the ligand in ethanol was added to the obtained solutions. The complexes were obtained in high yield (>90%).
The microstructural parameters of the complexes (the composition and structure of the nearest spheres around the iron atom) were obtained from EXAFS spectroscopy data. The selection of the oscillating part of the absorption spectrum (EXAFS functions) from the total spectrum was carried out using the VIPER 10.17 program [40]. The radial distribution functions around the iron atom were obtained by the Fourier transform of k3-multiplied (weighted) EXAFS functions in the wavenumber range Δk = 2.0–13.0 Å–1 (Fig. 1).
The structure of the complex with 2,6-bis(benzimidazol-2-yl)pyridine was used as the initial model for calculating the spectra of complexes in the low-spin state due to the similarity of the structures of the coordination site of the iron(II) ion with that and the ligand studied in this work (2.6-bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine) and the assumption that two ligands are coordinated by the iron(II) ion according to the tridentate cyclic type. The simulation of the local environment around the iron atom (interatomic distances Ri, atomic coordinates, angles) was carried out using the EXCURVE 98 program [41] for the whole molecule in the multiple scattering approximation without taking into account hydrogen atoms, CH3 groups, and anions, since they have little effect on the shape EXAFS spectrum due to structural remoteness from the central iron atom. This made it possible to reduce the number of variable parameters in the simulation process. The simulation procedure was carried out in the range of wave vectors Δk = 3.0–12 Å–1 with the weighting coefficient w = k3 and the value of the amplitude suppression factor S02 = 1.0. The structure of the coordination site and the data on the local environment of the iron atom, obtained by modeling the EXAFS spectra are shown in Fig. 2 and in Table 1, respectively.
For comparison, Fig. 3 shows the experimental and model spectra of complex [FeL2]B10H10·2H2O in the HS state.
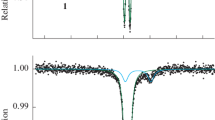
Measurements of the absorption spectra for complexes in the high-spin state were only possible for complex [FeL2]B12H12·H2O with a lower transition temperature. Figure 4 shows the XANES spectra in the region of the Fe K-edge absorption and the first derivatives for the complex in the LS and HS states.
As can be seen from Fig. 4, when the complex is heated to 420 K, the absorption edge shifts to lower energies by 1.5 eV due to the shift of unoccupied states and an increase in interatomic distances from the central iron atom to neighboring (Fe–N) atoms [37, 42]. This is due to the fact that the strength of the crystal field of the ligand in the low-spin state of the complex is higher than in the high-spin state.
For complex [FeL2]B12H12·H2O in the HS state, the EXAFS spectrum was simulated in a single approximation for the spectrum filtered in real space (ΔR = 1.0–3.2 Å). The coordination numbers of the nearest spheres of the environment around the iron atom are fixed in accordance with the octahedral structure of the coordination site of the complex obtained by modeling the EXAFS spectrum of the complex in the LS state in the multiple scattering approximation. The averaged data on the local structure around the iron atom for complex [FeL2]B12H12·H2O in the LS and HS states are given in Table 2, and a comparison of the model and experimental spectra for this complex in the HS state is shown in Fig. 5.
Table 3 shows the main vibrational frequencies of L and complexes 1 and 2. In the spectra of the complexes in the region of 3600–3500 cm–1, bands of the O–H stretching vibrations are recorded. In the spectrum of L in the range 3460–3200 cm–1, there are broad weakly resolved bands of stretching vibrations of NH groups, which are included in hydrogen bonds. In the spectra of the complexes, the ν(NH) bands noticeably shift (3250 (1) and 3291 cm–1 (2)) as compared to the spectrum of L and become clearer, which is probably due to the weakening of hydrogen bonds upon complexation. The ν(CH) and ν(CH3) bands are observed in the range of 3200–2800 cm–1, while the B–H stretching vibration bands are observed in the range of 2480–2430 cm–1. The number and position of the bands of stretching and bending vibrations of the rings in the spectra of complexes 1 and 2 change in comparison with the spectrum of L, which indicates the coordination of the nitrogen atoms of the heterocycles by iron(II). This is also confirmed by the data of spectra of 1 and 2 in the long-wave region, where metal–ligand stretching vibrations are manifested. Here, the bands at 294 and 295 cm–1 are observed, which are absent in the ligand spectrum and belong to the Fe–N stretching vibrations (Table 3).
The diffuse reflection spectra of complexes 1·2H2O and 2·H2O contains absorption bands, which are related to the 1А1 → 1Т2 and 1А1 → 1Т1 transitions in the strong octahedral field of the ligands. The spectra of both complexes lack the 5Т2 → 5Е band, which refers to the high-spin state of iron(II). As a result, we calculated the splitting parameters from the difference between the absorption frequencies 1А1 → 1Т2 and 1А1 → 1Т1 [43]. B values were calculated using the formula: 16B = [ν(1А1 → 1Т2) – ν(1А1 → 1Т1)]. To calculate the values of C and ΔLS given in Table 4, the following approximations were used: νLS = ΔLS – C + 86B2/ΔLS; С = 4.41В [44, 45]. The obtained data are close to the calculated values of ΔLS for the low-spin iron(II) complexes with 2,6-bis(benzimidazol-2-yl)pyridine and a number of outer-sphere anions that we obtained earlier [25, 26]. This indicates that 2,6-bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine is a strong field ligand. The calculated values of the splitting parameters correspond to the inequality, which is the condition for the manifestation of the spin-crossover [44]: 19 000 ≤ ΔLS ≤ 22 000 cm–1.
The Mössbauer spectra of both complexes are quadrupole doublets (Fig. 6, Table 5), the parameters of which correspond to the LS state of iron.
When comparing the parameters of low-spin doublets with similar parameters of Fe(II) compounds with 2,6-bis(benzimidazol-2-yl)pyridine, which we studied earlier [26], one should note an increase in both chemical shifts and quadrupole splittings. It can be assumed that the increase in chemical shifts is associated with an increase in the density of iron 3d electrons due to the higher electron density on nitrogen atoms coordinated by Fe(II) in the case of complexes with 2,6-bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine. An increase in the quadrupole splittings is apparently due to an increase in the electric field gradient on the iron nuclei due to a decrease in the distance from the outer-sphere anions to iron(II), associated with a decrease in the size of the ligand.
The temperature dependences χT of the studied complexes and their dehydrated analogs are shown in Figs. 7, 8. Spin crossover is observed both for the synthesized phase of complex [FeL2]B10H10⋅2H2O and for its dehydrate. The value of χT for [FeL2]B10H10⋅2H2O in the empirically selected temperature range of complex stability reaches ~1.53 K cm3/mol. The complex does not completely transform into the high-spin state (χTcalcd = 3 K cm3/mol) upon heating to 520 K. Crystallization water does not significantly affect the χT values achieved in the considered temperature range. At the same time, the residual value of χT in the low-spin state for the dehydrated complex increases to 0.21 K cm3/mol compared to 0.1 K cm3/mol for the initial [FeL2]B10H10⋅2H2O. The residual value χT ≠ 0 may be due to the presence of temperature-independent paramagnetism or partial “unfreezing” of the orbital angular momentum. Despite the incomplete spin-crossover, the condition d2(µeff(T))/dT2 = 0 is satisfied in the studied temperature range, which makes it possible to determine the transition temperatures (Figs. 7c, 7d). The temperatures of direct (Тс↑) and reverse (Тс↓) transitions for [FeL2]B10H10⋅2H2O are presented in Table 6. It can be seen that the transition temperatures during dehydration somewhat decrease, while the hysteresis does not change significantly. Thus, the dehydration of complex [FeL2]B10H10⋅2H2O most significantly affects the temperature of the direct and reverse transitions.
Unlike the previous complexes, for [FeL2]B12H12⋅H2O and [FeL2]B12H12 a complete spin crossover is observed (Fig. 8). It should be noted that, compared with the previous pair of complexes, the observed temperatures of the direct spin-crossover (Tc↑) are lower by 110–177 K (Table 6). The value of χT for these complexes in the high-spin state is 2.88 K cm3/mol, which is close to the theoretical value of 3.0 K cm3/mol for Fe(II) in the high-spin state and agrees with the experimental values of 2.76–3.92 K cm3/mol for transition metal complexes with configuration 3d6 [46]. In the low spin state, the residual magnetic moment is 0.03 K cm3/mol for [FeL2]B12H12⋅H2O and 0.18 K cm3/mol for [FeL2]B12H12. Thus, the presence of crystallization water, as in the case of [FeL2]B10H10⋅2H2O and [FeL2]B10H10, causes lower values of the residual magnetic moment. For complex [FeL2]B12H12⋅H2O, the presence of hysteresis can be noted on the χT(Т) dependence curve (8°, Table 6). When passing to the dehydrated complex [FeL2]B12H12, a decrease in the transition temperature is observed, and there is no hysteresis on the curve. Thus, the crystallization water for [FeL2]B12H12⋅H2O and [FeL2]B12H12 has a more significant effect on the spin-crossover parameters than in the case of the previous pair of complexes.
CONCLUSIONS
New iron(II) complexes with 2,6-bis(4,5-dimethyl-1Н-imidazol-2-yl)pyridine and closo-borate anions [FeL2]B10H10⋅2H2O and [FeL2]B12H12⋅H2O were synthesized. It was shown that both the starting compounds and their dehydrated analogs have a thermally induced spin-crossover 1А1 ↔ 5Т2. It should be noted that the replacement of the \({{{\text{B}}}_{{10}}}{\text{H}}_{{10}}^{{2 - }}\) anion by \({{{\text{B}}}_{{12}}}{\text{H}}_{{12}}^{{2 - }}\) in the composition of dehydrated complexes leads to a change in the direct spin crossover temperature (Tc↑) by 177 K. The comparison was made for the dehydrates of the complexes in order to eliminate the influence of solvate water molecules. In addition, we note the general trend towards a decrease in the temperatures of the forward and reverse transitions and an increase in the residual magnetic moment upon removal of crystallization water for complexes [FeL2]B10H10⋅2H2O and [FeL2]B12H12⋅H2O.
REFERENCES
Spin Crossover in Transition Metal Compounds I–III, Ed. by P. Gutlich and H. Goodwin (Springer, 2004).
M. A. Halcrow, Spin-Crossover Materials Properties and Applications (Wiley & Sons, 2013).
O. Kahn, J. Krober, and C. Jay, Adv. Mater. 4, 718 (1992). https://doi.org/10.1002/adma.19920041103
O. G. Shakirova and L. G. Lavrenova, Crystals 10, 843 (2020). https://doi.org/10.3390/cryst10090843
M. Shatruk, H. Phan, B. A. Chrisostomo, and A. Suleimenova, Coord. Chem. Rev. 289, 62 (2015). https://doi.org/10.1016/j.ccr.2014.09.018
H. L. C. Feltham, A. S. Barltrop, and S. Brooker, Coord. Chem. Rev. 344, 26 (2017). https://doi.org/10.1016/j.ccr.2016.10.006
H. S. Scott, R. W. Staniland, and P. E. Kruger, Coord. Chem. Rev. 362, 24 (2018). https://doi.org/10.1016/j.ccr.2018.02.001
E. K. Melnikova, D. Yu. Aleshin, I. A. Nikovskiy, et al., Crystals 10, 793 (2020). https://doi.org/10.3390/cryst10090793
L. G. Lavrenova, Russ. Chem. Bull. Int. Ed. 67, 1142 (2018). https://doi.org/10.1007/s11172-018-2195-3
P. Gamez, J. S. Costa, M. Quesada, and G. Aromi, Dalton Trans. 7845 (2009). https://doi.org/10.1039/B908208E
S. Hayami, S. M. Holmes, and M. A. Halcrow, J. Mater. Chem. 3, 7775. https://doi.org/10.1039/C5TC90128F
M. Matsuda, H. Isozaki, and H. Tajima, Chem. Lett. 37, 374 (2008). https://doi.org/10.1246/cl.2008.374
R. N. Muller, V. Elst, and S. Laurent, J. Am. Chem. Soc. 125, 8405 (2003). https://doi.org/10.1021/ja0349599
J.-F. Letard, N. Daro, C. Aymonier, et al., EP Patent 2391631 (2011).
A. Bousseksou, C. Vieu, J.-F. Letard, et al., EU Patent 1430552 (2004).
L. G. Lavrenova and S. V. Larionov, Russ. J. Coord. Chem. 24, 379 (1998).
L. G. Lavrenova and O. G. Shakirova, Eur. J. Inorg. Chem. 670 (2013). https://doi.org/10.1002/ejic.201200980
L. G. Lavrenova, O. G. Shakirova, V. N. Ikorskii, et al., Russ. J. Coord. Chem. 29, 22 (2003). https://doi.org/10.1023/A:1021834715674
L. G. Lavrenova, V. N. Ikorskii, V. A. Varnek, et al., Koord. Khim. 16, 654 (1990).
M. Piedrahita-Bello, J. E. Angulo-Cervera, R. Courson, et al., J. Mater. Chem. C 8, 6001 (2020). https://doi.org/10.1039/D0TC01532F
T. D. Nguyen, J. M. Veauthier, G. F. Angles-Tamayo, et al., JACS 142, 4842 (2020). https://doi.org/10.1021/jacs.9b13835
M. Boča, R. F. Jameson, and W. Linert, Coord. Chem. Rev. 255, 290 (2011). https://doi.org/10.1016/j.ccr.2010.09.010
R. Boča, P. Baran, M. Boca, et al., Inorg. Chim. Acta 278, 190 (1998). https://doi.org/10.1016/S0020-1693(98)00023-1
L. G. Lavrenova, I. I. Dyukova, E. V. Korotaev, et al., Russ. J. Inorg. Chem. 65, 30 (2020). https://doi.org/10.1134/S0036023620010106
A. D. Ivanova, E. V. Korotaev, V. Yu. Komarov, et al., New J. Chem. 44, 5834 (2020). https://doi.org/10.1039/D0NJ00474J
A. D. Ivanova, L. G. Lavrenova, E. V. Korotaev, et al., Russ. J. Inorg. Chem. 65, 1687 (2020). https://doi.org/10.1134/S0036023620110078
L. J. K. Cook, R. Mohammed, G. Sherborne, et al., Coord. Chem. Rev. 289, 2 (2015). https://doi.org/10.1016/j.ccr.2014.08.006
A. A. Pavlov, D. Yu. Aleshin, I. A. Nikolskiy, et al., Eur. J. Inorg. Chem. 23, 2819 (2019). https://doi.org/10.1002/ejic.201900432
V. García-López, M. Palacios-Corella, V. Gironés-Pérez, et al., Inorg. Chem. 58, 12199 (2019). https://doi.org/10.1021/acs.inorgchem.9b01526
A. D. Ivanova, E. V. Korotaev, V. Yu. Komarov, et al., Inorg. Chim. Acta 532, 120746 (2022). https://doi.org/10.1016/j.ica.2021.120746
I. B. Sivaev, V. I. Bregadze, and N. T. Kuznetsov, Izv. Akad. Nauk. Ser. Khim. 8, 1256 (2002).
M. F. Hawthorne, Mol. Med. Today 4, 174 (1998). https://doi.org/10.1016/S1357-4310(98)01226-X
Y. Zhu, Y. Lin, Y. Z. Zhu, et al., J. Nanomater. 2010 (2010). https://doi.org/10.1155/2010/409320
R. A. Spryshkova, Doctoral Sci. (Biol.) Dissertation, Moscow, 1999.
M. F. Hawthorne, Angew. Chem. Int. Ed. Eng. 32, 950 (1993). https://doi.org/10.1002/anie.199309501
M. B. Bushuev, L. G. Lavrenova, Yu. G. Shvedenkov, et al., Russ. J. Coord. Chem. 34, 190 (2008). https://doi.org/10.1134/S107032840803007X
O. G. Shakirova, V. A. Daletskii, L. G. Lavrenova, et al., Russ. J. Inorg. Chem. 58, 650 (2013). https://doi.org/10.1134/S0036023613060211
H. C. Miller and E. L. Muetterties, Inorg. Synth. 10, 81 (1967).
P. A. Piminov, G. N. Baranov, A. V. Bogomyakov, et al., Phys. Proc. 84, 19 (2016). https://doi.org/10.1016/j.phpro.2016.11.005
K. V. Klementiev, J. Phys. D: Appl. Phys. 34, 209 (2001). https://doi.org/10.1088/0022-3727/34/2/309
N. Binsted, J. W. Campbell, S. J. Gurman, and P. C. Stephenson, SERC Daresbury Laboratory Report (1991).
V. G. Vlasenko, S. P. Kubrin, D. A. Garnovskii, et al., Chem. Phys. Lett. 739, 136970 (2020). https://doi.org/10.1016/j.cplett.2019.136970
E. Liver, Electronic Spectroscopy of Inorganic Compounds (Mir, Moscow, 1987) [in Russian].
A. Hauser, Top Curr. Chem. 233, 49 (2004). https://doi.org/10.1007/b13528
S. Sugano, Y. Tanabe, and H. Kamimura, Multiplets of Transition-Metal Ions in Crystals (Academic Press Pure and Applied Physics, London, New York, 1970).
Yu. V. Rakitin and V. T. Kalinnikov, Modern Magnetochemistry (Nauka, St. Petersburg, 1994).
ACKNOWLEDGMENTS
The authors are grateful to the Chemical Research Center for Collective Use of the Siberian Branch of the Russian Academy of Sciences for the spectral and analytical measurements.
Funding
The work was supported by the Russian Science Foundation (grant no. 20-63-46026) and the Ministry of Science and Higher Education of the Russian Federation, projects nos. 121031700313-8, 121031700314-5, and 1021051403061-8-1.4.1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ADDITIONAL INFORMATION
The article was prepared based on the materials of the XXVIII International Chugaev Conference on Coordination Chemistry, Ol’ginka, Tuapse oblast, Russia, October 3–8, 2021.
Additional information
Translated by V. Avdeeva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ivanova, A.D., Lavrenova, L.G., Korotaev, E.V. et al. Study of Spin-Crossover in Iron(II) Complexes with 2,6-Bis(4,5-Dimethyl-1H-Imidazol-2-yl)Pyridine and closo-Borate Anions. Russ. J. Inorg. Chem. 67, 1158–1168 (2022). https://doi.org/10.1134/S0036023622080174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622080174