Abstract
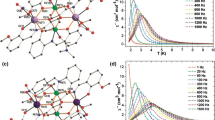
New iron(II) complexes with 2,6-bis(benzimidazol-2-yl)pyridine (L) and closo-borate(2–) anions—[FeL2][B10H10] ⋅ 2H2O and [FeL2][B12H12] ⋅ H2O—have been synthesized. The complexes have been characterized by static magnetic susceptibility measurements, electronic (diffuse reflectance spectra), IR, and EXAFS spectroscopy, and X-ray diffraction analysis. A study of the μeff(T) dependence in the temperature range 80–500 K has shown that the complexes exhibit a high-temperature spin crossover 1А1 ⇔ 5Т2.








Similar content being viewed by others
REFERENCES
Spin Crossover in Transition Metal Compounds I–III, Ed. by P. Gütlich and H. Goodwin (Springer, 2004).
M. A. Halcrow, Spin-Crossover Materials Properties and Applications (Wiley, 2013).
O. Kahn, J. Kröber, and C. Jay, Adv. Mater. 4, 718 (1992).
L. G. Lavrenova and O. G. Shakirova, Eur. J. Inorg. Chem, 670 (2013).
M. Shatruk, H. Phan, B. A. Chrisostomo, and A. Suleimenova, Coord. Chem. Rev. 289–290, 62 (2015).
H. L. C. Feltham, A. S. Barltrop, and S. Brooker, Coord. Chem. Rev. 344, 26 (2017).
H. S. Scott, R. W. Staniland, and P. E. Kruger, Coord. Chem. Rev. 362, 24 (2018).
O. G. Shakirova, L. G. Lavrenova, A. S. Bogomyakov, et al., Russ. J. Inorg. Chem. 60, 786 (2015).
E. N. Frolova, T. A. Ivanova, O. A. Turanova, et al. Russ. J. Inorg. Chem. 63, 1012 (2018).
P. Gamez, J. S. Costa, M. Quesada, and G. Aromí, Dalton Trans., 7845 (2009).
S. Hayami, S. M. Holmes, and M. A. Halcrow, J. Mater. Chem. C 3, 7775 (2015).
M. Matsuda, H. Isozaki, and H. Tajima, Chem. Lett. 37, 374 (2008).
R. N. Muller, V. Elst, and S. Laurent, J. Am. Chem. Soc. 125, 8405 (2003).
J.-F. Letard, N. Daro, C. Aymonier, et al., Patent EP2391631 (2011).
A. Bousseksou, C. Vieu, J.-F. Letard, et al., EU Patent 1 430 552 (2004).
M. Boča, R. F. Jameson, and W. Linert, Coord. Chem. Rev. 255, 290 (2011).
R. Boča, P. Baran, M. Boča, et al., Inorg. Chim. Acta 278, 190 (1998).
L. G. Lavrenova, I. I. Dyukova, E. V. Korotaev, et al., Russ. J. Inorg. Chem. 65, 30 (2020).
A. D. Ivanova, E. V. Korotaev, V. Yu. Komarov, et al., New J. Chem. 44, 5834 (2020).
I. B. Sivaev, V. I. Bregadze, and N. T. Kuznetsov, Izv. Akad. Nauk, Ser. Khim., No. 8, 1256 (2002).
M. F. Hawthorne, Mol. Med. Today 4, 174 (1998).
Y. Zhu, Y. Lin, Y. Z. Zhu, et al., J. Nanomater. 2010, art. 409 320 (2010).
V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 62, 1673 (2017).
S. E. Korolenko, V. V. Avdeeva, E. A. Malinina, et al., Russ. J. Coord. Chem. 46, 297 (2020).
O. G. Shakirova, L. G. Lavrenova, and V. N. Ikorskiy, et al., Chem. Sust. Dev. 10, 757 (2002).
M. B. Bushuev, L. G. Lavrenova, Yu. G. Shvedenkov, et al., Russ. J. Coord. Chem. 34, 190 (2008).
O. G. Shakirova, V. A. Daletskii, L. G. Lavrenova, et al., Russ. J. Inorg. Chem. 58, 650 (2013).
H. C. Miller and E. L. Muetterties, Inorg. Synth. 10, 81 (1967).
A. Lever, Inorganic Electronic Spectroscopy (Elsevier, Amsterdam, 1984; Mir, Moscow, 1987).
A. Hauser, Top Curr. Chem. 233, 49 (2004).
S. Sugano, Y. Tanabe, and H. Kamimura, Multiplets of Transition-Metal Ions in Crystals (Academic Press Pure and Applied Physics, New York, 1970).
ACKNOWLEDGMENTS
We are grateful to N.P. Korotkevich for recording the X‑ray powder diffraction patterns and to I.V. Yushina for recording the diffuse reflectance spectra.
Funding
The work was partially supported by the Russian Science Foundation (project no. 20-63-46026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Ivanova, A.D., Lavrenova, L.G., Korotaev, E.V. et al. High-Temperature Spin Crossover in Complexes of Iron(II) closo-Borates with 2,6-Bis(benzimidazol-2-yl)pyridine. Russ. J. Inorg. Chem. 65, 1687–1694 (2020). https://doi.org/10.1134/S0036023620110078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620110078