Abstract
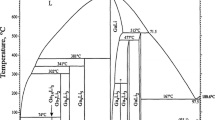
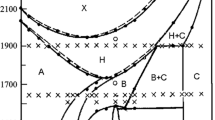
The phase diagrams of binary systems of gallium sulfate with lithium or sodium sulfate were studied for the first time. The Li2SO4–Ga2(SO4)3 system is of the eutectic type. The coordinates of the eutectic are (548°C, 30 mol % Ga2(SO4)3). The region of a solid solution based on the high-temperature modification α-Li2SO4 is small. In the Na2SO4–Ga2(SO4)3 system, compound Na3Ga(SO4)3 forms, which melts incongruently at 585°C. The coordinates of the eutectic are (538°C, 17 mol % Ga2(SO4)3). The region of a solid solution based on α-Na2SO4 reaches 8 ± 1 mol % Ga2(SO4)3. The X-ray powder diffraction pattern of Na3Ga(SO4)3 was indexed in a tetragonal unit cell with the parameters a = 9.451(3) Å and c = 7.097(3) Å; the unit cell parameters for an aluminum-containing analog, Na3Al(SO4)3, are a = 9.424(5) Å and c = 7.053(3) Å.
Similar content being viewed by others
References
M. R. Palacin, Chem. Soc. Rev. 38, 2565 (2009).
B. Dunn, H. Kamath, and J. M. Tarascon, Science 1334, 928 (2011).
J. B. Goodenough, Acc. Chem. Rev. 16, 1053 (2013).
A. B. Bykov, A. P. Chirkin, L. N. Demyanets, et al., Solid State Ionics 38, 31 (1990).
Y. Wang, H. Li, M. Chen, et al., Ionics 23, 377 (2017).
X. Xiang, K. Zhang, and J. Chen, Adv. Mater. 27, 5343 (2015).
P. Barpanda, G. Oyama, C. D. Ling, and A. Yamada, Chem. Mater. 26, 1297 (2014).
A. Manthiram and J. B. Goodenough, J. Power Sources 26, 403 (1989).
R. V. Ivanova, Chemistry and Technology of Gallium (Metallurgiya, Moscow, 1973) [in Russian].
P. I. Fedorov, M. V. Mokhosoev, and F. P. Alekseev, Chemistry of Gallium, Indium, and Thallium (Nauka, Novosibirsk, 1977) [in Russian].
M. Krause and R. Gruehn, Z. Kristallogr. 210, 427 (1995).
S. P. Yatsenko, Zh. Neorg. Khim. 6, 1922 (1961).
P. I. Fedorov and Zhang Chi-yuin, Zh. Neorg. Khim. 11, 669 (1966).
R. Fricke and W. Blencke, Z. Anorg. Allg. Chem. 143, 183 (1925).
P. A. Kokkoros, Tscher. Miner. Petrogr. 10, 45 (1965).
Handbuch der Präparativen Anorganischen Chemie, Ed. by G. Brauer (Ferdinand Enke, Stuttgart, 1975).
Yu. V. Koryakin and I. I. Angelov, Pure Chemical Substances: A Handbook of Preparation of Inorganic Reagents and Substances (Khimiya, Moscow, 1974) [in Russian].
V. Yu. Proidakova, S. V. Kuznetsov, V. V. Voronov, and P. P. Fedorov, Fine Chemical Technologies. 12 (3), 52 (2017).
P. I. Fedorov, P. P. Fedorov, and D. V. Drobot, Physicochemical Analysis of Anhydrous Salt Systems, (MIKhM–MITKhT, Moscow, 1987) [in Russian].
P. P. Fedorov and L. V. Medvedeva, Zh. Neorg. Khim. 34, 2674 (1989).
R. Perret, J. Tudo, B. Jolibois, and P. Couchot, J. Less-Common Met. 37, 9 (1974).
A. F. Polishchuk, Zh. Fiz. Khim. 18, 1930 (1973).
P. P. Fedorov, Russ. J. Inorg. Chem. 45 (Suppl. 3), S268 (2000).
P. P. Fedorov, B. P. Sobolev, and P. I. Fedorov, Sov. Phys. Crystallogr. 26, 291 (1981).
P. P. Fedorov, Solid State Ionics 84, 113 (1996).
N. K. Voskresenskaya, N. N. Evseeva, S. I. Berul’, and I. P. Vereshchetina, A Handbook of Melting of Anhydrous Inorganic Salt Systems, Vol. 1: Binary Systems (Akad. Nauk SSSR, Moscow, 1961) [in Russian].
K. A. Bol’shakov, P. I. Fedorov, and N. I. Il’ina, Zh. Neorg. Khim. 8, 2577 (1963).
W. Eysel, H. H. Hofer, K. L. Keester, and Th. Hahn, Acta Crystallogr., Sect. B 41, 5 (1985).
P. P. Fedorov, T. M. Polkhovskaya, B. P. Sobolev, et al., Sov. Phys. Crystallogr. 28, 353 (1983).
A. N. Pokrovskii, Doctoral Dissertation in Chemistry (MGU, Moscow, 1981).
P. I. Fedorov and P. P. Fedorov, Russ. J. Inorg. Chem. 46, 1422 (2001).
A. Lunden, Fast Ion Transp. Solids 250, 181 (1993).
R. D. Shannon, Acta Crystallogr., Sect. A, 32, 751 (1976).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.P. Fedorov, V.Yu. Proidakova, S.V. Kuznetsov, V.V. Voronov, 2017, published in Zhurnal Neorganicheskoi Khimii, 2017, Vol. 62, No. 11, pp. 1515–1520.
Rights and permissions
About this article
Cite this article
Fedorov, P.P., Proidakova, V.Y., Kuznetsov, S.V. et al. Phase equilibria in systems of gallium sulfate with lithium or sodium sulfate. Russ. J. Inorg. Chem. 62, 1508–1513 (2017). https://doi.org/10.1134/S0036023617110067
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617110067