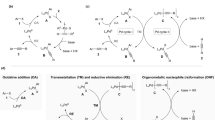
Abstract
The paper presents the results of a comparative study of the differential selectivity of the copper- and ligand-free Sonogashira reaction under so-called artificial multiroutness aimed at distinguishing between homogeneous and heterogeneous catalysis mechanisms. The use of different amounts of soluble and insoluble heterogeneous palladium catalyst precursors resulted in the same values of the differential reaction selectivity for competing aryl iodides, competing arylacetylenes, and their conversion products. The observed patterns are consistent with the occurrence of the Sonogashira reaction in solution through a homogeneous catalysis mechanism, in particular, in the presence of insoluble heterogeneous catalyst precursors.


Similar content being viewed by others
REFERENCES
Rayadurgam, J., Sana, S., Sasikumar, M., and Gu, Q., Org. Chem. Front., 2021, vol. 8, no. 2, p. 384.
Heravi, M.M., Ghanbarian, M., Ghalavand, N., and Nazari, N., Curr. Org. Chem., 2018, vol. 22, no. 14, p. 1420.
Martek, B.A., Gazvoda, M., Urankar, D., and Košmrlj, J., Org. Lett., 2020, vol. 22, no. 13, p. 4938.
Leyva-Pérez, A., Oliver-Meseguer, J., Rubio-Marqués, P., and Corma, A., Angew. Chem. Int. Ed., 2013, vol. 52, no. 44, p. 11554.
Djakovitch, L. and Rollet, P., Adv. Synth. Catal., 2004, vol. 346, nos. 13–15, p. 1782.
Ananikov, V.P. and Beletskaya, I.P., Organometallics, 2012, vol. 31, p. 1595.
Prima, D.O., Kulikovskaya, N.S., Galushko, A.S., Mironenko, R.M., and Ananikov, V.P., Curr. Opin. Green Sustain. Chem., 2021, vol. 31, p. 100502.
Eremin, D.B. and Ananikov, V.P., Coord. Chem. Rev., 2017, vol. 346, p. 2.
Biffis, A., Centomo, P., del Zotto, A., and Zecca, M., Chem. Rev., 2018, vol. 118, p. 2249.
Schmidt, A.F., Kurokhtina, A.A., and Larina, E.V., Kinet. Catal., 2012, vol. 53, p. 84.
Schmidt, A.F., Kurokhtina, A.A., and Larina, E.V., Kinet. Catal., 2019, vol. 60, p. 551.
Billo, E.J., Excel for Scientists and Engineers: Numerical Methods, New York: Wiley, 2007.
Mironenko, R.M., Belskaya, O.B., and Likholobov, V.A., Russ. J. Gen. Chem., 2020, vol. 90, p. 532.
Mikhaylov, V.N., Sorokoumov, V.N., Liakhov, D.M., Tskhovrebov, A.G., and Balova, I.A., Catalysts, 2018, vol. 8, no. 4, p. 141.
Zhao, X., Liu, X., Zhu, Y., and Lu, M., Appl. Organomet. Chem., 2015, vol. 29, no. 10, p. 674.
Genelot, M., Dufaud, V., and Djakovitch, L., Adv. Synth. Catal., 2013, vol. 355, p. 2604.
Thathagar, M.B., Kooyman, P.J., Boerleider, R., Jansen, E., Elsevier, C.J., and Rothenberg, G., Adv. Synth. Catal., 2005, vol. 347, no. 15, p. 1965.
Dubey, P. and Singh, A.K., ChemistrySelect, 2020, vol. 5, no. 10, p. 2925.
Goncalves, R.S.B., de Oliveira, A.B.V., Sindra, H.C., Archanjo, B.S., Mendoza, M.E., Carneiro, L.S.A., Buarque, C.D., and Esteves, P.M., ChemCatChem, 2016, vol. 8, no. 4, p. 743.
Ezugwu, C.I., Mousavi, B., Asrafa, A., Mehta, A., Vardhan, H., and Verpoort, F., Catal. Sci. Technol., 2016, vol. 6, no. 7, p. 2050.
Kuchkina, N.V., Sorokina, S.A., Bykov, A.V., Sulman, M.G., Bronstein, L.M., and Shifrina, Z.B., Nanomater, 2021, vol. 11, no. 12, p. 3345.
Alapour, S., Farahani, M.D., Ramjugernath, D., Koorbanally, N.A., and Friedrich, H.B., ACS Sustain. Chem. Eng., 2019, vol. 7, no. 15, p. 12697.
Gholinejad, M., Esmailoghli, H., Khosravi, F., and Sansano, J.M., J. Organomet. Chem., 2022, vol. 963, p. 122295.
Karami, K., Abedanzadeh, S., Afroomand, M., Herves, P., and Bayat, P., Catal. Lett. (in press).
Widegren, J.A. and Finke, R.G., J. Mol. Catal. A: Chem., 2003, vol. 198, p. 317.
Crabtree, R.H., Chem. Rev., 2012, vol. 112, no. 3, p. 1536.
Schmidt, A.F. and Kurokhtina, A.A., Kinet. Catal., 2012, vol. 53, p. 714.
Köhler, K., Kleist, W., and Pröckl, S.S., Inorg. Chem., 2007, vol. 46, p. 1876.
Schmidt, A.F., al Halaiqa, A., and Smirnov, V.V., Synlett, 2006, vol. 18, p. 2861.
Wussow, K., Abram, A., and Kohler, K., Catal. Commun., 2022, vol. 165, p. 106441.
Finke, R.G. and Ozkar, S., J. Phys. Chem. C, 2019, vol. 123, p. 54.
Siemsen, P., Livingston, R.C., and Diederich, F., Angew. Chem. Int. Ed., 2000, vol. 39, no. 15, p. 2632.
Temkin, O.N., Kinet. Catal., 2012, vol. 53, no. 3, p. 313.
Schmidt, A.F., Kurokhtina, A.A., and Larina, E.V., Catal. Sci. Technol., 2014, vol. 4, p. 3439.
Bandini, M., Luque, R., Budarin, V., and Macquarrie, D.J., Tetrahedron, 2005, vol. 61, no. 41, p. 9860.
Funding
This work was supported by the Russian Science Foundation (project no. 21-73-00137) and performed using the equipment of the Center for Collective Use of Analytical Instrumentation at the Irkutsk State University (http://ckp-rf.ru/ckp/3264/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Abbreviations and notation: DMF, N,N-dimethylformamide; DS, differential selectivity.
Rights and permissions
About this article
Cite this article
Larina, E.V., Kurokhtina, A.A., Lagoda, N.A. et al. Discrimination between the Homogeneous and Heterogeneous Mechanisms of Catalysis in the Copper- and Ligand-Free Sonogashira Reaction Using Phase Trajectory Analysis. Kinet Catal 64, 431–438 (2023). https://doi.org/10.1134/S0023158423040055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158423040055