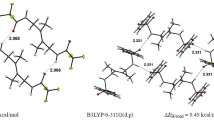
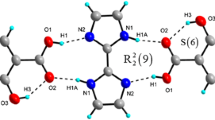
Abstract
The title compound, 1,1′-((1E,1′E)-(pyridine-3,4-diylbis(azanylylidene))bis(methanylylidene))bis (naphthalen-2-ol) (1), was synthesized and structurally characterized. The compound cocrystallized with one MeCN molecule. Interestingly, one of two salicylaldehyde Schiff base fragments exists in enol form, while the other one - in a ketone form. Moreover, cocrystallized acetonitrile molecule forms hydrogen bonding with three hydrogen atoms of the dye molecule. The nature and energies of intermolecular and intramolecular hydrogen bonds were studied theoretically using DFT calculations and topological analysis of the electron density distribution within the formalism of Bader′s theory (QTAIM method).






Similar content being viewed by others
REFERENCES
O. V. Repina, A. S. Novikov, O. V. Khoroshilova, A. S. Kritchenkov, A. A. Vasin, and A. G. Tskhovrebov. Inorg. Chim. Acta, 2020, 502, 119378. https://doi.org/10.1016/j.ica.2019.119378
A. G. Tskhovrebov, A. S. Novikov, O. V. Odintsova, V. N. Mikhaylov, V. N. Sorokoumov, T. V. Serebryanskaya, and G. L. Starova. J. Organomet. Chem., 2019, 886, 71-75. https://doi.org/10.1016/j.jorganchem.2019.01.023
M. D. Cohen and G. M. J. Schmidt. J. Phys. Chem., 1962, 66(12), 2442-2446. https://doi.org/10.1021/j100818a030
A. G. Tskhovrebov, E. Solari, R. Scopelliti, and K. Severin. Organometallics, 2014, 33(10), 2405-2408. https://doi.org/10.1021/om500333y
V. I. Minkin, A. V. Tsukanov, A. D. Dubonosov, and V. A. Bren. J. Mol. Struct., 2011, 998(1-3), 179-191. https://doi.org/10.1016/j.molstruc.2011.05.029
M. Sliwa, N. Mouton, C. Ruckebusch, L. Poisson, A. Idrissi, S. Aloïse, L. Potier, J. Dubois, O. Poizat, and G. Buntinx. Photochem. Photobiol. Sci., 2010, 9(5), 661. https://doi.org/10.1039/b9pp00207c
M. Sliwa, N. Mouton, C. Ruckebusch, S. Aloïse, O. Poizat, G. Buntinx, R. Métivier, K. Nakatani, H. Masuhara, and T. Asahi. J. Phys. Chem. C, 2009, 113(27), 11959-11968. https://doi.org/10.1021/jp901849a
A. A. Astafiev, O. V. Repina, B. S. Tupertsev, A. A. Nazarov, M. R. Gonchar, A. V. Vologzhanina, V. G. Nenajdenko, A. S. Kritchenkov, V. N. Khrustalev, V. N. Nadtochenko, and A. G. Tskhovrebov. Molecules, 2021, 26(6), 1739. https://doi.org/10.3390/molecules26061739
A. G. Tskhovrebov, A. S. Novikov, B. S. Tupertsev, A. A. Nazarov, A. A. Antonets, A. A. Astafiev, A. S. Kritchenkov, A. S. Kubasov, V. G. Nenajdenko, and V. N. Khrustalev. Inorg. Chim. Acta, 2021, 522, 120373. https://doi.org/10.1016/j.ica.2021.120373
A. G. Tskhovrebov, A. A. Vasileva, R. Goddard, T. Riedel, P. J. Dyson, V. N. Mikhaylov, T. V. Serebryanskaya, V. N. Sorokoumov, and M. Haukka. Inorg. Chem., 2018, 57(3), 930-934. https://doi.org/10.1021/acs.inorgchem.8b00072
D. R. Williams. Chem. Rev., 1972, 72(3), 203-213. https://doi.org/10.1021/cr60277a001
A. Wodajo, A. G. Tskhovrebov, T. A. Le, A. S. Kubasov, M. M. Grishina, O. N. Krutius, and V. N. Khrustalev. Acta Crystallogr., Sect. E: Crystallogr. Commun., 2020, 76(10), 1579-1581. https://doi.org/10.1107/S2056989020012104
A. G. Tskhovrebov, A. S. Novikov, and V. N. Khrustalev. J. Struct. Chem., 2021, 62(3), 460-466. https://doi.org/10.1134/S0022476621030136
J.-D. Chai and M. Head-Gordon. Phys. Chem. Chem. Phys., 2008, 10(44), 6615. https://doi.org/10.1039/b810189b
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Had, and D. J. Fox. Gaussian09. Wallingford CT: Gaussian, Inc., 2010.
R. F. W. Bader. Chem. Rev., 1991, 91(5), 893-928. https://doi.org/10.1021/cr00005a013
T. Lu and F. Chen. J. Comput. Chem., 2012, 33(5), 580-592. https://doi.org/10.1002/jcc.22885
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. Stalke. J. Appl. Crystallogr., 2015, 48(1), 3-10. https://doi.org/10.1107/S1600576714022985
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64(1), 112-122. https://doi.org/10.1107/S0108767307043930
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/S2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42(2), 339-341. https://doi.org/10.1107/S0021889808042726
E. Espinosa, E. Molins, and C. Lecomte. Chem. Phys. Lett., 1998, 285(3/4), 170-173. https://doi.org/10.1016/S0009-2614(98)00036-0
M. V. Vener, A. N. Egorova, A. V. Churakov, and V. G. Tsirelson. J. Comput. Chem., 2012, 33(29), 2303-2309. https://doi.org/10.1002/jcc.23062
I. Rozas, I. Alkorta, and J. Elguero. J. Am. Chem. Soc., 2000, 122(45), 11154-11161. https://doi.org/10.1021/ja0017864
T. Steiner. Angew. Chem., Int. Ed., 2002, 41(1), 48-76. https://doi.org/10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
V. N. Mikhaylov, V. N. Sorokoumov, A. S. Novikov, M. V. Melnik, A. G. Tskhovrebov, and I. A. Balova. J. Organomet. Chem., 2020, 912, 121174. https://doi.org/10.1016/j.jorganchem.2020.121174
V. Mikhaylov, V. Sorokoumov, D. Liakhov, A. Tskhovrebov, and I. Balova. Catalysts, 2018, 8(4), 141. https://doi.org/10.3390/catal8040141
N. G. Shikhaliyev, A. M. Maharramov, G. T. Suleymanova, A. A. Babazade, V. G. Nenajdenko, V. N. Khrustalev, A. S. Novikov, and A. G. Tskhovrebov. Mendeleev Commun., 2021, 31(5), 677-679. https://doi.org/10.1016/j.mencom.2021.09.028
I. V. Buslov, A. S. Novikov, V. N. Khrustalev, M. V. Grudova, A. S. Kubasov, Z. V. Matsulevich, A. V. Borisov, J. M. Lukiyanova, M. M. Grishina, A. A. Kirichuk, T. V Serebryanskaya, A. S. Kritchenkov, and A. G. Tskhovrebov. Symmetry (Basel), 2021, 13(12), 2350. https://doi.org/10.3390/sym13122350
M. V. Grudova, V. N. Khrustalev, A. S. Kubasov, P. V. Strashnov, Z. V. Matsulevich, J. M. Lukiyanova, G. N. Borisova, A. S. Kritchenkov, M. M. Grishina, A. A. Artemjev, I. V. Buslov, V. K. Osmanov, V. G. Nenajdenko, N. Q. Trung, A. V Borisov, and A. G. Tskhovrebov. Cryst. Growth Des., 2021, acs.cgd.1c00954. https://doi.org/10.1021/acs.cgd.1c00954
V. N. Khrustalev, M. M. Grishina, Z. V. Matsulevich, J. M. Lukiyanova, G. N. Borisova, V. K. Osmanov, A. S. Novikov, A. A. Kirichuk, A. V. Borisov, E. Solari, and A. G. Tskhovrebov. Dalton Trans., 2021, 50(31), 10689-10691. https://doi.org/10.1039/D1DT01322J
E. Espinosa, I. Alkorta, J. Elguero, and E. Molins. J. Chem. Phys., 2002, 117(12), 5529-5542. https://doi.org/10.1063/1.1501133
E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen, and W. Yang. J. Am. Chem. Soc., 2010, 132(18), 6498-6506. https://doi.org/10.1021/ja100936w
J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan, and W. Yang. J. Chem. Theory Comput., 2011, 7(3), 625-632. https://doi.org/10.1021/ct100641a
Funding
Funding for this research was provided by RFBR (project number 20-53-00006) and Belarusian Foundation for Fundamental Research (grant X20P-066).
This work was supported by the RUDN University Strategic Academic Leadership Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 4, pp. 510-512.https://doi.org/10.26902/JSC_id91711
Supplementary material
Rights and permissions
About this article
Cite this article
Mardaleishvili, I.R., Vologzhanina, A.V., Novikov, A.S. et al. HYDROGEN BONDING IN THE CRYSTAL OF 1,1′-((1E,1′E)-(PYRIDINE-3,4-DIYLBIS (AZANYLYLIDENE))BIS(METHANYLYLIDENE))- BIS(NAPHTHALEN-2-OL) ACETONITRILE SOLVATE: COMBINED EXPERIMENTAL AND THEORETICAL STUDY. J Struct Chem 63, 626–633 (2022). https://doi.org/10.1134/S002247662204014X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002247662204014X