Abstract
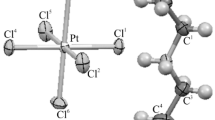
A new complex of Pt(II) bearing N-(bis(2,4-dimethoxybenzyl)carbamothioyl)-4-methylbenzamide ligand is prepared and characterized by 1H, 13C, HMQC, COSY NMR and FTIR spectroscopic techniques. The structure of the complex is determined by single crystal X-ray diffraction. The structural data reveal that the ligands in the cis-conformation attach to the platinum center and the complex has a slightly distorted square-planar geometry. The molecular packing of the complex is mainly supported by the C–H⋯O hydrogen bond, the C–H⋯π interaction, and Pt⋯H nonclassical interactions. The three-dimensional (3D) Hirshfeld surfaces and the associated 2D fingerprint plots of the complex are investigated for the intermolecular hydrogen bonding and non-covalent interactions. In addition, the antimicrobial activity of the free ligand and the Pt(II) complex is evaluated against Gram-positive bacteria (S. aureus, S. pneumoniae, E. faecalis, B. subtilis), Gram-negative bacteria (E. coli, P. aeruginosa), and fungal strains (C. albicans, C. glabrata). The results of this evaluation reveal that ligand is much more active against bacteria than the Pt(II) complex. The highest dose found to be effective against bacterial and fungal strains is evaluated in terms of cytotoxic effects on peripheral blood lymphocytes. It is determined that the 500 μg/mL dose does not cause a cytotoxic effect.









Similar content being viewed by others
REFERENCES
E. Dixon and J. Hawthorne. J. Chem. Soc., 1907, 91, 122. https://doi.org/10.1039/CT9079100122
B. Douglass and F. B. Dains. J. Am. Chem. Soc., 1934, 56, 719. https://doi.org/10.1021/ja01318a057
A. N. Westra, S. A. Bourne, C. Esterhuysen, and K.R. Koch. Dalton Trans., 2005, 2162. https://doi.org/10.1039/b503653d
Z. Weiqun, Y. Wen, Q. Lihua, Z. Yong, and Y. Zhengfeng. J. Mol. Struct., 2005, 749, 89. https://doi.org/10.1016/j.molstruc.2005.03.046
S. Bozkurt, I. Gumus, and H. Arslan. J. Organomet. Chem., 2019, 884, 66. https://doi.org/10.1016/j.jorganchem.2019.01.015
F. M. Emen, U. Flörke, N. Külcü, and H. Arslan. Acta Crystallogr., Sect. E: Struct. Rep. Online, 2003, 59, m582. https://doi.org/10.1107/S1600536803014934
G. Avşar, H. Arslan, H.-J. Haupt, and N. Külcü. Turk. J. Chem., 2003, 27, 281.
H. Arslan, D. Vanderveer, F. Emen, and N. Külcü. Z. Kristallogr. – New Cryst. Struct., 2003, 218, 479. https://doi.org/10.1524/ncrs.2003.218.4.479
S. Orysyk, V. Bon, and V. Pekhnyo. Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, m1059. https://doi.org/10.1107/S1600536809029262
K. R. Koch. Coord. Chem. Rev., 2001, 473, 216. https://doi.org/10.1016/S0010-8545(01)00337-X
A. Saeed, U. Flörke, and M. F. Erben. J. Sulfur Chem., 2014, 35, 318. https://doi.org/10.1080/17415993.2013.834904
K.-H. König, M. Schuster, B. Steinbrech, G. Schneeweis, and R. Schlodder. Fresenius Z. Anal. Chem., 1985, 321, 457. https://doi.org/10.1007/BF00487079
M. Merdivan. Talanta, 2000, 53, 141. https://doi.org/10.1016/S0039-9140(00)00464-1
M. Schuster, B. Kugler, and K.-H. König. Fresenius Z. Anal. Chem., 1990, 338, 717. https://doi.org/10.1007/BF00323412
M. Schuster and M. Sandor. Anal. Bioanal. Chem., 1996, 356, 326. https://doi.org/10.1007/s0021663560326
S. Mihai and M. Negoiu. Rev. Chim., 2012, 63, 697.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339. https://doi.org/10.1107/S0021889808042726
L. Palatinus and G. Chapuis. J. Appl. Crystallogr., 2007, 40, 786. https://doi.org/10.1107/S0021889807029238
L. Palatinus and A. van der Lee. J. Appl. Crystallogr., 2008, 41, 975. https://doi.org/10.1107/S0021889808028185
L. Palatinus, S. J. Prathapa, and S. van Smaalen. J. Appl. Crystallogr., 2012, 45, 575. https://doi.org/10.1107/S0021889812016068
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
I. Gumus, U. Solmaz, G. Binzet, E. Keskin, B. Arslan, and H. Arslan. J. Mol. Struct., 2018, 1157, 78. https://doi.org/10.1016/j.molstruc.2017.12.017
I. Gumus, S. Gonca, B. Arslan, E. Keskin, U. Solmaz, and H. Arslan. Eur. J. Chem., 2017, 8, 410. https://doi.org/10.5155/eurjchem.8.4.410-416.1650
I. Gumus, U. Solmaz, G. Binzet, E. Keskin, B. Arslan, and H. Arslan. J. Mol. Struct., 2019, 1180, 542. https://doi.org/10.1016/j.molstruc.2018.12.024
U. Solmaz, I. Gumus, G. Binzet, O. Celik, G. K. Balci, A. Dogen, and H. Arslan. J. Coord. Chem., 2018, 71, 200. https://doi.org/10.1080/00958972.2018.1427233
I. Gumus, U. Solmaz, O. Celik, G. Binzet, G. K. Balcı, and H. Arslan. Eur. J. Chem., 2015, 6, 237. https://doi.org/10.5155/eurjchem.6.3.237-241.1265
H. Jorgensen and M. J. Ferraro. Clin. Infect. Dis., 1998, 26, 973. https://doi.org/10.1086/513938
A. W. Bauer, W. M. M. Kirby, J. C. Serris, and M. Turck. Am. J. Clin. Pathol., 1966, 45, 493. https://doi.org/10.1093/ajcp/45.4_ts.493
Manual of Clinical Microbiology / Eds. J. H. Jorgensen, K. C. Carroll, G. Funke, M. A. Pfaller, M. L. Landry, S. S. Richter, and D. W. Warnock. Washington, DC: ASM Press, 2015. https://doi.org/10.1128/9781555817381
P. S. Moorhead, P. C. Nowell, W. J. Mellman, D. M. Battips, and D. A. Hungerford. Exp. Cell Res., 1960, 20, 613. https://doi.org/10.1016/0014-4827(60)90138-5
E. Y. Bekfelavi, P. Küce Çevik, Ö. Yılmaz, N. Ş. Kuş, G. Coral, and A. Çelik. Bioorg. Chem. Med. Rep., 2018, 1, 6. https://doi.org/10.25135/bmcr.12.18.11.1035
D. A. Eastmond and J. D. Tucker. Environ. Mol. Mutgen., 1989, 13, 34. https://doi.org/10.1002/em.2850130104
A. OReilly, A. M. Plutin, H. Pérez, O. Calderón, R. Ramos, R. Martinez, R. A. Toscano, J. Duque, H. Rodriguez-Solla, R. Martinez-Alvarez, M. Suarez, and N. Martin. Polyhedron, 2012, 36, 133. https://doi.org/10.1002/ejic.200400864
E. Keskin, U. Solmaz, G. Binzet, I. Gumus, and H. Arslan. Eur. J. Chem., 2018, 9, 360. https://doi.org/10.5155/eurjchem.9.4.360-368.1774
S. Roy, M. G. B. Drew, A. Bauzá, A. Frontera, and S. Chattopadhyay. Dalton Trans., 2017, 46, 5384. https://doi.org/10.1039/C6DT04906K
Y.-F. Jiang, C.-J. Xi, Y.-Z. Liu, J. Niclós-Gutiérrez, and D. Choquesillo-Lazarte. Eur. J. Inorg. Chem., 2005, 2005, 1585. https://doi.org/10.1002/ejic.200400864
O. Kroutil, M. Předota, and Z. Chval. Inorg. Chem., 2016, 55, 3252. https://doi.org/10.1021/acs.inorgchem.5b02261
M. A. Spackman and D. Jayatilaka. CrystEngComm, 2009, 11, 19. https://doi.org/10.1039/B818330A
M. Keikha, M. Pourayoubi, A. Tarahhomi, and A. van der Lee. Z. Kristallogr. - Cryst. Mater., 2017, 232, 453. https://doi.org/10.1515/zkri-2016-2032
I. Gumus, U. Solmaz, S. Gonca, and H. Arslan. Eur. J. Chem., 2017, 8, 349. https://doi.org/10.5155/eurjchem.8.4.349-357.1637
N. T. Abdel-Ghani and A. M. Mansour. J. Coord. Chem., 2012, 65, 763. https://doi.org/10.1080/00958972.2012.661048
M. Eweis, S. S. Elkholy, and M. Z. Elsabee. Int. J. Biol. Macromol., 2006, 38, 1. https://doi.org/10.1016/j.ijbiomac.2005.12.009
G. H. Fleet. Curr. Top. Med. Mycol., 1985, 1, 24. https://doi.org/10.1007/978-1-4613-9547-8_2
M. Fenech. Mutat. Res., 2006, 600, 58. https://doi.org/10.1016/j.mrfmmm.2006.05.028
Funding
This work was supported by the Mersin University Research Fund (Project No: 2015-AP4-1162).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 1, pp. 33-35.https://doi.org/10.26902/JSC_id85833
Supplementary material
Rights and permissions
About this article
Cite this article
Solmaz, U., Keskin, E., Gumus, I. et al. PLATINUM(II) COMPLEX CONTAINING N-(BIS (-2,4-DIMETHOXY-BENZYL)CARBAMOTHIOYL)- 4-METHYLBENZAMIDE LIGAND: SYNTHESIS, CRYSTAL STRUCTURE, HIRSHFELD SURFACE ANALYSIS, AND ANTIMICROBIAL ACTIVITY. J Struct Chem 63, 62–74 (2022). https://doi.org/10.1134/S0022476622010073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622010073