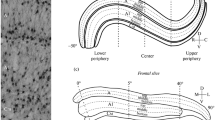
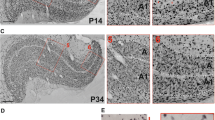
Abstract
We carried out a comparative morphometric analysis of neurons in the cat dorsal lateral geniculate nucleus (dLGN) in frontal vs. sagittal slices. Using the SMI-32 antibody to non-phosphorylated domains of heavy-chain neurofilaments, the postnatal dynamic of soma parameters (area, roundness, orientation) of dLGN neurons was studied. Measurements were performed in kittens aged 0, 4, 10, 14, 21, 28, 34, 62, 123 days, and in adult cats. A comparison of data obtained in frontal vs. sagittal slices revealed the following significant differences: (1) the soma area of the immunopositive neurons was smaller in frontal vs. sagittal slices in all age groups, and this difference increased with age; (2) the soma orientation was also different in two cutting planes, and a significant age-related change in the soma orientation occurred only in the sagittal, but not frontal, plane. We assume that the difference in the soma area is due to the spatial arrangement of SMI-32-immunopositive neurons in the dLGN, because of which, in the sagittal plane, in contrast to the frontal, neuronal somas were cut parallel to their long axis. In turn, age-related changes in the soma orientation may reflect an age-related internal rearrangement of the dLGN retinotopic organization.

Similar content being viewed by others
REFERENCES
Oberlaender M (2019) Neuronal Morphology and Its Significance. In: Singer W, Sejnowski TJ, Rakic P (eds) The Neocortex. The MIT Press, Cambridge, Massachusetts, pp 124–138.
Hendry SHC, Jones EG (1983) The organization of pyramidal and non-pyramidal cell dendrites in relation to thalamic afferent terminations in the monkey somatic sensory cortex. J Neurocytol 12:277–298. https://doi.org/10.1007/BF01148465
Herculano-Houzel S, Manger PR, Kaas JH (2014) Brain scaling in mammalian evolution as a consequence of concerted and mosaic changes in numbers of neurons and average neuronal cell size. Front Neuroanat 8:1–28. https://doi.org/10.3389/fnana.2014.00077
Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF (2009) Pharmacological inhibition of mTORCl suppresses anatomical, cellular, and behavioral abnormalities in neural-specific PTEN knock-out mice. J Neurosci 29:1773–1783. https://doi.org/10.1523/JNEUROSCI.5685-08.2009
Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ (1996) Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA 93:11202–11207. https://doi.org/10.1073/pnas.93.20.11202
Loopuijt LD, Villablanca JR, Sharifi P (2001) Soma size of substantia nigra neurons increases after a prenatal neocortical lesion in cats. Dev Brain Res 130:143–147. https://doi.org/10.1016/S0165-3806(01)00223-1
Duffy KR, Slusar JE (2009) Monocular deprivation provokes alteration of the neuronal cytoskeleton in developing cat lateral geniculate nucleus. Vis Neurosci 26:319–28. https://doi.org/10.1017/S0952523809090130
Flood DG, Coleman PD (1988) Neuron numbers and sizes in aging brain: Comparisons of human, monkey, and rodent data. Neurobiol Aging 9:453–463. https://doi.org/10.1016/S0197-4580(88)80098-8
Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36. https://doi.org/10.1007/s00401-009-0532-1
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HBM, Friedman L, Rajkowska G (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56:640–650. https://doi.org/10.1016/j.biopsych.2004.08.022
Rajković K, Marić DL, Milošević NT, Jeremic S, Arsenijević VA, Rajković N (2016) Mathematical modeling of the neuron morphology using two dimensional images. J Theor Biol 390:80–85. https://doi.org/10.1016/j.jtbi.2015.11.019
FitzGibbon T (2006) Does the development of the perigeniculate nucleus support the notion of a hierarchical progression within the visual pathway? Neuroscience 140:529–546. https://doi.org/10.1016/j.neuroscience.2006.02.038
Acciai L, Soda P, Iannello G (2016) Automated Neuron Tracing Methods: An Updated Account. Neuroinformatics 14:353–367. https://doi.org/10.1007/s12021-016-9310-0
Garman RH, Li AA, Kaufmann W, Auer RN, Bolon B (2016) Recommended Methods for Brain Processing and Quantitative Analysis in Rodent Developmental Neurotoxicity Studies. Toxicol Pathol 44:14–42. https://doi.org/10.1177/0192623315596858
Burnat K, Van Der Gucht E, Waleszczyk WJ, Kossut M, Arckens L (2012) Lack of early pattern stimulation prevents normal development of the alpha (Y) retinal ganglion cell population in the cat. J Comp Neurol 520:2414–2429. https://doi.org/10.1002/cne.23045
Merkulyeva NS, Mikhalkin AAA, Veshchitskii AAA, Merkul’eva NS, Mikhalkin AAA, Veshchitskii AAA (2016) Characteristics of the Distribution of Acetylcholinesterase in the Posterolateral Nucleus of the Thalamus in Cats. Neurosci Behav Physiol 46:507–509. https://doi.org/10.1007/s11055-016-0267-0
Mooser F, Bosking WH, Fitzpatrick D (2004) A morphological basis for orientation tuning in primary visual cortex. Nat Neurosci 7:872–879. https://doi.org/10.1038/nn1287
Yan C, Li A, Zhang B, Ding W, Luo Q, Gong H (2013) Automated and Accurate Detection of Soma Location and Surface Morphology in Large-Scale 3D Neuron Images. PLoS One 8:1–12. https://doi.org/10.1371/journal.pone.0062579
Radojević M, Meijering E (2019) Automated Neuron Reconstruction from 3D Fluorescence Microscopy Images Using Sequential Monte Carlo Estimation. Neuroinformatics 17:423–442. https://doi.org/10.1007/s12021-018-9407-8
Lima D, Coimbra A (1986) A Golgi study of the neuronal population of the marginal zone (lamina I) of the rat spinal cord. J Comp Neurol 244:53–71. https://doi.org/10.1002/cne.902440105
Merkulyeva N, Veshchitskii A, Makarov F, Gerasimenko Y, Musienko P (2016) Distribution of 28 kDa calbindin-immunopositive neurons in the cat spinal cord. Front Neuroanat 9:166. https://doi.org/10.3389/fnana.2015.00166
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A 80:6126–6130. https://doi.org/10.1073/pnas.80.19.6126
Nurzynska K, Mikhalkin A, Piorkowski A (2017) CAS: Cell Annotation Software—Research on Neuronal Tissue Has Never Been so Transparent. Neuroinformatics 15:365–382. https://doi.org/10.1007/s12021-017-9340-2
Piorkowski A, Piórkowski A (2016) A statistical dominance algorithm for edge detection and segmentation of medical images. Adv Intell Syst Comput 471:3–14. https://doi.org/10.1007/978-3-319-39796-2_1
Kutcher MR, Duffy KR (2007) Cytoskeleton alteration correlates with gross structural plasticity in the cat lateral geniculate nucleus. Vis Neurosci 24:775–785. https://doi.org/10.1017/S095252380707068X
Quené H, Van Den Bergh H (2004) On multi-level modeling of data from repeated measures designs: A tutorial. Speech Commun 43:103–121. https://doi.org/10.1016/j.specom.2004.02.004
Mikhalkin A, Nikitina N, Merkulyeva N (2020) Heterochrony of postnatal accumulation of nonphosphorylated heavy‐chain neurofilament by neurons of the cat dorsal lateral geniculate nucleus. J Comp Neurol 529(7):1–12. https://doi.org/10.1002/cne.25028
Feller MB, Scanziani M (2005) A precritical period for plasticity in visual cortex. Curr Opin Neurobiol 15:94–100. https://doi.org/10.1016/j.conb.2005.01.012
Eysel UT, Wolfhard U (1983) Morphological fine tuning of retinotopy within the cat lateral geniculate nucleus. Neurosci Lett 39:15–20. https://doi.org/10.1016/0304-3940(83)90158-1
Bishop PO, Kozak W, Levick WR, Vakkur GJ (1962) The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol 163:503–539. https://doi.org/10.1113/jphysiol.1962.sp006991
Sanderson KJ (1971) The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol 143:101–117. https://doi.org/10.1002/cne.901430107
Stanford LR, Friedlander MJ, Sherman SM (1983) Morphological and physiological properties of geniculate W-cells of the cat: a comparison with X- and Y-cells. J Neurophysiol 50:582–608. https://doi.org/10.1152/jn.1983.50.3.582
Friedlander MJ, Lin CS, Stanford LR, Sherman SM (1981) Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol 46:80–129. https://doi.org/10.1152/jn.1981.46.1.80
Bickford ME, Guido W, Godwin DW (1998) Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: Normal expression and alteration with visual deprivation. J Neurosci 18:6549–6557. https://doi.org/10.1523/JNEUROSCI.18-16-06549.1998
Duffy KR, Crowder NA, LeDue EE (2012) Investigation of cytoskeleton proteins in neurons of the cat lateral geniculate nucleus. J Comp Neurol 520:186–199. https://doi.org/10.1002/cne.22727
Kalil R (1978) Development of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 182:265–291. https://doi.org/10.1002/cne.901820206
Elgeti H, Elgeti R, Fleischhauer K (1976) Postnatal growth of the dorsal lateral geniculate nucleus of the cat. Anat Embryol (Berl) 149:1–13. https://doi.org/10.1007/BF00315081
Coleman LA, Friedlander MJ (2002) Postnatal dendritic development of Y-like geniculocortical relay neurons. Int J Dev Neurosci 20:137–159. https://doi.org/10.1016/S0736-5748(02)00018-7
Coleman LA, Friedlander MJ (1992) Intracellular injections of permanent tracers in the fixed slice: a comparison of HRP and biocytin. J Neurosci Methods 44:167–177. https://doi.org/10.1016/0165-0270(92)90009-3
Friedlander MJ (1982) Structure of physiologically classified neurones in the kitten dorsal lateral geniculate nucleus. Nature 300:180–183. https://doi.org/10.1038/300180a0
ACKNOWLEDGMENT
The authors are grateful to N.I. Nikitina for her assistance in microscopic studies.
Funding
This work was supported by the Governmental Program 47 “Scientific and Technological Development of the Russian Federation” for 2019–2030, theme 0134-2019-0006 (theoretical part) and the Russian Science Foundation grant No. 21-15-00235 (experimental part).
Author information
Authors and Affiliations
Contributions
A concept and experimental design: A.A.M. and N.S.M.; data collection and processing: A.A.M.; statistical data treatment: A.A.M.; data analysis and interpretation: A.A.M. and N.S.M.; manuscript writing: A.A.M. and N.S.M.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest, both evident and potential, as related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2021, Vol. 57, No. 5, pp. 373–379https://doi.org/10.31857/S0044452921050053.
Rights and permissions
About this article
Cite this article
Mikhalkin, A.A., Merkulyeva, N.S. Peculiarities of Age-Related Dynamics of Neurons in the Cat Lateral Geniculate Nucleus as Revealed in Frontal versus Sagittal Slices. J Evol Biochem Phys 57, 1001–1007 (2021). https://doi.org/10.1134/S0022093021050021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021050021