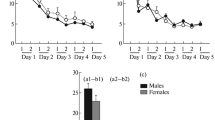
Abstract
In adolescent rats (25–35-day-old) exposed at birth (neonatal days 1 and, repeatedly, 2) to adverse impacts (inflammatory pain, short-term maternal separation stress, or both), sexual dimorphism was found in pain behavior under identical peripheral inflammation conditions. Our priority data indicate an enhancement of pain response in the formalin test in males, whereas in females pain sensitivity did not change, i.e. pain experienced in females at birth did not affect the system reactivity to the same chemical irritant in the adolescent period. However, rats of both sexes exposed to short-term maternal deprivation stress (60 min on neonatal days 1 and 2) exhibited the enhanced pain sensitivity in the formalin test. The combined impact of inflammatory pain and maternal deprivation in neonatal pups did not change pain sensitivity at adolescence both in males and females. Male and female rats exposed to early maternal deprivation exhibited decreased anxiety levels in the elevated plus-maze; rats exposed to each of the above-mentioned early impacts showed a decline in adaptive behavior in the forced swim test; males exposed to pain and combined impacts demonstrated the spatial learning impairment in the Morris labyrinth. Thus, we have pioneered in demonstrating sex-dependent differences in the effect of inflammatory pain in neonatal rat pups on the inflammatory pain sensitivity in adolescent rats. The division of early stress and pain impacts was revealed in adolescent females in the formalin test: while maternal deprivation induced hyperalgesia, pain did not change the tonic nociceptive system functional activity.
Similar content being viewed by others
References
Kassil, V.G., Otellin, V.A., Khozhai, L.I., and Kostkin, V.B., Critical phases of the brain development, Ross. Fiziol. Zh. im. I.M. Sechenova, 2000, vol. 86, no. 11, pp. 1418–1425.
Rice, D. and Barone, S., ffixJr., Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models, Environ. Health Perspect., 2000, vol. 108, suppl 3, pp. 511–533.
Horvath, G., Reglodi, D., Farkas, J., Vadasz, G., Mammel, B., Kvarik, T., Bodzai, G., Kiss-Illes, B., Farkas, D., Matkovits, A., Manavalan, S., Gaszner, B., Tamas, A., and Kiss, P., Perinatal positive and negative influences on early neurobehavioral reflex and motor development, Adv. Neurobiol., 2015, vol. 10, pp. 149–67.
Knaepen, L., Patijn, J., van Kleef, M., Mulder, M., Tibboel, D., and Joosten, E.A.J., Neonatal repetitive needle pricking: plasticity of the spinal nociceptive circuit and extended postoperative pain in later life, Dev. Neurobiol., 2013, vol. 73, pp. 85–97.
Walker, S.M., Biological and neurodevelopmental implications of neonatal pain, Clin. Perinatol., 2013, vol. 40, pp. 471–91.
Zouikr, I., Tadros, M.A., Barouei, J., Beagley, K.W., Clifton, V.L., Callister, R.J., and Hodgson, D.M., Altered nociceptive, endocrine, and dorsal horn neuron responses in rats following a neonatal immune challenge, Psychoneuroendocrinol., 2014, vol. 41, pp. 1–12.
Anand, K.J., Palmer, F.B., and Papanicolaou, A.C., Repetitive neonatal pain and neurocognitive abilities in ex-preterm children, Pain, 2013, vol. 154, pp. 1899–901.
Grunau, R.E., Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity, Rambam Maimonides Med. J., 2013, vol. 4(4):e0025.doi: 10.5041/RMMJ.10132.
Walker, S.M., Neonatal pain, Paediatr. Anaesth., 2014, vol. 24, pp. 39–48.
Krugers, H.J. and Joëls, M., Long-lasting Consequences of Early Life stress on Brain Structure, Emotion and Cognition, Curr. Top. Behav. Neurosci., 2014, vol. 18, pp. 81–92.
Victoria, N.C., Karom, M.C., Eichenbaum, H., and Murphy, A.Z., Neonatal injury alters markers of pain and stress in rat pups, Dev. Neurobiol., 2014, vol. 74, pp. 42–51.
Zhang, Y.H, Wang, X.M, and Ennis, M., Effects of neonatal inflammation on descending modulation from the rostroventromedial medulla, Brain Res. Bull., 2010, vol. 83, pp. 16–22.
Ranger, M. and Grunau, R.E., Early repetitive pain in preterm infants in relation to the developing brain, Pain Manag., 2014, vol. 4, pp. 57–67.
Schwaller, F. and Fitzgerald, M., The consequences of pain in early life: injury-induced plasticity in developing pain pathways, Eur. J. Neurosci., 2014, vol. 39, pp. 344–52.
LaPrairie, J.L. and Murphy, A.Z., Long term impact of neonatal injury in male and female rats: sex differences, mechanisms and clinical implications, Front. Neuroendocrinol., 2010, vol. 31, pp. 193–202.
Ruda, M.A., Ling, Q.D., Hohmann, A.G., Peng, Y.B., and Tachibana, T., Altered nociceptive neuronal circuits after neonatal peripheral inflammation, Science, 2000, vol. 289, pp. 628–631.
Bhutta, A.T., Rovnaghi, C., Simpson, P.M., Gossett, J.M., Scalzo, F.M., and Anand, K.J.S., Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects, Physiol. Behav., 2001, vol. 73, pp. 51–58.
Lidov, M.S., Long-term effects of neonatal pain on nociceptive systems, Pain, 2002, vol. 99, pp. 337–383.
Ren, K., Anseloni, V., Zou, S.P., Wade, E.B., Novikova, S.I., Ennis, M., Traub, R.J., Gold, M.S., Dubner, R., and Lidov, M.S., Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult, Pain, 2004, pp. 110, pp. 588–596.
Negrigo, A., Medeiros, M., Guinsburg, R., and Covolan, L., Long-term gender behavioral vulnerability after nociceptive neonatal formalin stimulation in rats, Neurosci. Lett., 2011, vol. 190, pp. 196–199.
Dubuisson, D. and Dennis, S.G., The formalin test: a quantitative study of the analgesic effects of morphine, meperidine and brain stimulation in rats and cats, Pain, 1977, vol. 4, pp. 161–174.
Barrot, M., Tests and models of nociception and pain in rodents, Neuroscience, 2012, vol. 211, pp. 39–50.
Johnston, C.C. and Walker, C.-D., The effects of exposure to repeated minor pain during the neonatal period on formalin pain behavior and thermal withdrawal latencies, Pain Res. Manag., 2003, vol. 8, pp. 213–217.
Fillingim, R.B., King, C.D., Ribeiro-Dasilva, M.C., Rahim-Williams, B., and Riley 3rd, J.L., Sex, gender, and pain: a review of recent clinical and experimental findings, J. Pain, 2009, vol. 10, pp. 447–485.
Aloisi, A.M. and Sorda, G., Relationship of female sex hormones with pain perception: focus on estrogens, Pain Manag., 2011, vol. 1, pp. 229–238.
Hashmi, J.A. and Davis, K.D., Deconstructing sex differences in pain sensitivity, Pain, 2014, vol. 155, pp. 10–13.
LaPrairie, J.L. and Murphy, A.Z., Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury, Pain, 2007, vol. 132, p. 124.
Lima, M., Malheiros, J., Negrigo, A., Tescarollo, F., Medeiros, M., Suchecki, D., Tannus, A., Guinsburg, R., and Covolan, L., Sex-related long-term behavioral and hippocampal cellular alterations after nociceptive stimulation throughout postnatal development in rats, Neuropharmacol., 2014, vol. 77, pp. 268–76.
Butkevich, I.P., Mikhailenko, V.A., Lavrova, J.A., and Ulanova, N.A., Repeated inflammatory pain syndrome in newborn male rats changes adaptive behavior during adolescent period of development, Ross. Fiziol. Zh. im. I.M. Sechenova, 2014, vol. 100, pp. 1241–1251.
Zimmerman, M., Committee for Research and Ethical Issues of the IASP, Ethical standards for investigations of experimental pain in animals, Pain, 1983, vol. 16, pp. 109–110.
Barr, G.A., Maturation of the biphasic behavioral and heart rate response in the formalin test, Pharmacol. Biochem. Behav., 1998, vol. 60, pp. 329–335.
Porsolt, R.D., LePichon, M., and Jalfre, M., Depression: a new animal model sensitive to antidepressant treatments, Nature, 1977, vol. 266, pp. 730–732.
Morris, R.G.M., Spatial localization does not require the presence of local cues, Learning and Motivation, 1981, vol. 12, pp. 239–260.
Anseloni, V.C., He, F., Novikova, S.I., Turnbach, R.M., Lidov, I.A., Ennis, M., and Lidov, M.S., Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult, Neurosci., 2005, vol. 131, pp. 635–645.
Li, J. and Baccei, M.L., Neonatal tissue damage facilitates nociceptive synaptic input to the developing superficial dorsal horn via NGF-dependent mechanisms, Pain, 2011, vol. 152, pp. 1846–55.
Anand, K.J.S. and Scalzo, F.M., Can adverse neonatal experiences alter brain development and subsequent behavior? Biol. Neonate, 2000, vol. 77, pp. 69–82.
Arnold, A.P. and Gorski, R.A., Gonadal steroid induction of structural sex differences in the central nervous system, Ann. Rev. Neurosci., 1984, vol. 7, pp. 413–442.
Weisz, J. and Ward, I.L., Plasma testosterone and progesterone titers of pregnant rats, their male and famale fetuses, and neonatal offspring, Endocrinol., 1980, vol. 106, pp. 306–316.
Amateau, S.K., Alt, J.J., Stamps, C.L., and Mc-Carthy, M.M., Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon, Endocrinol., 2004, vol. 145, pp. 2906–2917.
Dallman, M.F., Moments in time—the neonatal rat hypothalamo–pituitery–adrenal axis, Neuroendocrinol., 2000, vol. 141, pp. 1590–1592.
Uhelski, M.L. and Fuchs, P.N., Maternal separation stress leads to enhanced emotional responses to noxious stimuli in adult rats, Behav. Brain Res., 2010, vol. 21, pp. 208–212.
Nishi, M., Horii-Hayashi, N., and Sasagawa, T., Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents, Front. Neurosci., 2014, vol. 8, 166. doi: 10.3389/fnins..00166.
McCormick, C.M., Furcy, B.F., Child, M., Sawyer, M.J., and Donohue, S.M., Neonatal sex hormones have “organizational” effects on the hypothalamic–pituitary–adrenal axis of male rats, Develop. Brain Res., 1998, vol. 105, pp. 295–307.
Romeo, R.D. and McEwen, B.S., Stress and adolescent brain, Ann. NY Acad. Sci., 2006, vol. 1094, pp. 202–214.
Goel, N., Workman, J.L., Lee, T.T., Innala, L., and Viau, V., Sex differences in the HPA axis, Compr. Physiol., 2014, vol. 4, pp. 1121–1155.
Toufexis, D., Rivarola, M.A., Lara, H., and Viau, V., Stress and the reproductive axis, J. Neuroendocrinol., 2014, vol. 26, pp. 573–86.
Fitzgerald, M. and Koltzenburg, M., The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat cord, Brain Res., 1986, vol. 389, pp. 261–270.
Rhodes, D.L. and Liebeskind, J.C., Analgesia from rostral brain stem stimulation in the rat, Brain Res., 1978, vol. 143, pp. 521–532.
Bourke, C.H. and Neigh, G.N., Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic, Horm. Behav., 2011, vol. 60, pp. 112–120.
Li, Y.J., Peng, S.L., Wan, C.Q., Cao, L., and Li, Y.P., Chronic pain impairs spatial learning and memory ability and down-regulates Bcl-2 and BDNF mRNA expression in hippocampus of neonatal rats, Zhonghua Er. Ke. Za. Zhi., 2005, vol. 43, pp. 444–448.
Anand, K.J.S., Physiology of pain in infants and children, Ann. Nestle., 1999, vol. 57, pp. 7–18.
Narsinghani, U. and Anand, K.J.S., Developmental neurobiology of pain in neonatal rats, Lab. Animal., 2000, vol. 29, pp. 27–39.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.P. Butkevich, V.A. Mikhailenko, E.A. Vershinina, N.A. Ulanova, 2015, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2015, Vol. 51, No. 4, pp. 266—275.
Rights and permissions
About this article
Cite this article
Butkevich, I.P., Mikhailenko, V.A., Vershinina, E.A. et al. Differences in adaptive behaviors of adolescent male and female rats exposed at birth to inflammatory pain or stress. J Evol Biochem Phys 51, 305–315 (2015). https://doi.org/10.1134/S0022093015040067
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093015040067