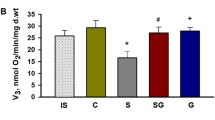
Abstract
Neuropeptide galanin and its N-terminal fragments reduce the generation of reactive oxygen species and normalize metabolic and antioxidant states of myocardium in experimental cardiomyopathy and ischemia/reperfusion injury. The aim of this study was to elucidate the effect of WTLNSAGYLLGPβAH-OH (peptide G), a pharmacological agonist of the galanin receptor GalR2, on the cardiac injury induced by administration of streptozotocin (STZ) in rats. Peptide G was prepared by solid phase peptide synthesis using the Fmoc strategy and purified by preparative HPLC; its structure was confirmed by 1H-NMR spectroscopy and MALDI-TOF mass spectrometry. Experimental animals were randomly distributed into five groups: C, control; S, STZ-treated; SG10, STZ + peptide G (10 nmol/kg/day); SG50, STZ + peptide G (50 nmol/kg/day); G, peptide G (50 nmol/kg/day). Administration of peptide G prevented hyperglycemia in SG50 rats. By the end of the experiment, the ATP content, total pool of adenine nucleotides, phosphocreatine (PCr) content, and PCr/ATP ratio in the myocardium of animals of the SG50 group were significantly higher than in rats of the S group. In the SG50 and SG10 groups, the content of lactate and lactate/pyruvate ratio in the myocardium were reduced, while the glucose content was increased vs. the S group. Both doses of peptide G reduced the activation of creatine kinase-MB and lactate dehydrogenase, as well as the concentration of thiobarbituric acid reactive products in the blood plasma of STZ-treated rats to the control values. Taken together, these results suggest that peptide G has cardioprotective properties in type 1 diabetes mellitus. Possible mechanisms of peptide G action in the STZ-induced diabetes are discussed.



Similar content being viewed by others
Abbreviations
- ΣAN:
-
total pool of adenine nucleotides (ATP + ADP + AMP)
- ΣCr:
-
total creatine (PCr + Cr)
- CK-MB:
-
creatine kinase MB
- DM:
-
diabetes mellitus
- GLUT4:
-
glucose transporter 4
- LDH:
-
lactate dehydrogenase
- LPO:
-
lipid peroxidation
- PCr:
-
phosphocreatine
- ROS:
-
reactive oxygen species
- STZ:
-
streptozotocin
- TBARS:
-
2-tiobarbituric acid reactive substances
- WTLNSAGYLL-NH2:
-
N-terminal fragment of galanin 2-11
- WTLNSAGYLLGPHA-OH:
-
N-terminal fragment of galanin 2-15
- WTLNSAGYLLGPβAH-OH (G):
-
modified analog of galanin 2-15
References
Varma, U., Koutsifeli, P., Benson, V. L., Mellor, K. M., and Delbridge, L. M. D. (2018) Molecular mechanisms of cardiac pathology in diabetes – experimental insights, Biochim. Biophys. Acta, 1864, 1949-1959, https://doi.org/10.1016/j.bbadis.2017.10.035.
Boudina, S., and Dale Abel, E. (2007) Diabetic cardiomyopathy revisited, Circulation, 115, 3213-3223, https://doi.org/10.1161/CIRCULATIONAHA.106.679597.
Verma, S. K., Garikipati, V. N. S., and Kishore, R. (2017) Mitochondrial dysfunction and its impact on diabetic heart, Biochim. Biophys. Acta, 1863, 1098-1105, https://doi.org/10.1016/j.bbadis.2016.08.021.
Maritim, A. C., Sanders, R. A., and Watkins, J. B. (2003) Diabetes, oxidative stress and antioxidants. A Review, J. Biochem. Mol. Toxicol., 17, 24-38, https://doi.org/10.1002/jbt.10058.
Ullah, A., Khan, A., and Khan, I. (2016) Diabetes mellitus and oxidative stress – a concise review, Saudi Pharmaceut. J., 24, 547-553, https://doi.org/10.1016/j.jsps.2015.03.013.
Raza, H., Prabu, S. K., John, A., and Avadhani, N. G. (2011) Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats, Internat. J. Mol. Sci., 12, 3133-3147, https://doi.org/10.3390/ijms12053133.
Webling, K. E. B., Runesson, J., Bartfai, T., and Langel, Ü. (2012) Galanin receptors and ligands, Front. Endocrinol., 3, 146, https://doi.org/10.3389/fendo.2012.00146.
Timotin, A., Pisarenko, O., Sidorova, M., Studneva, I., Shulzhenko, V., et al. (2017) Myocardial protection from ischemia/reperfusion injury by exogenous galanin fragment, Oncotarget, 8, 21241-21252, https://doi.org/10.18632/oncotarget.15071.
Pisarenko, O., Timotin, A., Sidorova, M., Studneva, I., Shulzhenko, V., et al. (2017) Cardioprotective properties of N-terminal galanin fragment (2-15) in experimental ischemia/reperfusion injury, Oncotarget, 8, 101659-101671, https://doi.org/10.18632/oncotarget.21503.
Serebryakova, L., Pal’keeva, M., Studneva, I., Molokoedov, A., Veselova, O., et al. (2019) Galanin and its N-terminal fragments reduce acute myocardial infarction in rats, Peptides, 111, 127-131, https://doi.org/10.1016/j.peptides.2018.05.001.
Pisarenko, O. I., Studneva, I. M., Serebryakova, L. I., Timoshin, A. A., Konovalova, G. G., et al. (2021) Antioxidant properties of galanin and its N-terminal fragments in in vitro and in vivo oxidative stress modeling, Biochemistry (Moscow), 86, 496-505, https://doi.org/10.1134/S0006297921040106.
Studneva, I., Palkeeva, M., Veselova, O., Molokoedov, A., Ovchinnikov, M., et al. (2019) Protective effects of a novel agonist of galanin receptors against doxorubicin-induced cardiotoxicity in rats, Cardiovasc. Toxicol., 19, 136-146, https://doi.org/10.1007/s12012-018-9483-x.
Studneva, I. M., Veselova, O. M., Bahtin, A. A., Konovalova, G. G., Lankin, V. Z., et al. (2020) The mechanisms of cardiac protection using a synthetic agonist of galanin receptors during chronic administration of doxorubicin, Acta Naturae, 12, 20-29, https://doi.org/10.32607/20758251-2020-12-1-89-98.
Fang, P., Sun, J., Wang. X., Zhang, Z., Bo, P., et al. (2013) Galanin participates in the functional regulation of the diabetic heart, Life Sci., 92, 628-632, https://doi.org/10.1016/j.lfs.2013.01.024.
He, B., Shi, M., Zhang, L., Li, G., Fang, P., et al. (2011) Beneficial effect of galanin on insulin sensitivity in muscle of type 2 diabetic rats, Physiol. Behav., 103, 284-289, https://doi.org/10.1016/j.physbeh.2011.02.023.
Legakis, I. N. (2005) The role of galanin in metabolic disorders leading to type 2 diabetes mellitus, Drug News Pers., 18, 173-177, https://doi.org/10.1358/dnp.2005.18.3.892762.
Az’muko, A. A., Veselova, O. M., Molokoedova, A. S., Ovchinnikov, M. V., Palkeeva, M. E., et al. (2018) Tetradecapeptides improving the reducing function of the cardiovascular system in ischemia, Patent no. 2648846.
Reaven, G. M., and Ho, H. (1991) Low-dose streptozotocin-induced diabetes in the spontaneously hypertensive rat, Metabolism, 40, 335-337, https://doi.org/10.1016/0026-0495(91)90141-i.
Bergmeyer, H. U. (1974) Methods of Enzymatic Analysis. New York, Academic Press, pp. 1196-1200, 1475-1478, 1772-1776, 1777-1781, 2101-2110.
Vanderlinde, R. E. (1985) Measurement of total lactate dehydrogenase activity, Ann. Clin. Lab. Sci., 15, 13-31.
Gulen, S., and Dincer, S. (2007) Effects of leptin on oxidative stress in healthy and streptozotocin-induced diabetic rats, Mol. Cell. Biochem., 302, 59-65, https://doi.org/10.1007/s11010-007-9426-5.
Stanley, W. C., Lopaschuk, G. D., James, G., and McCormack, J. G. (1997) Regulation of energy substrate metabolism in the diabetic heart, Cardiovasc. Res., 34, 25-33.
Saltiel, A. R., and Kahan, C. R. (2001) Insulin signaling and the regulation of glucose and lipid metabolism, Nature, 414, 799-806, https://doi.org/10.1038/414799a.
Dos Santos J. M., Tewari S., and Mendes R. H. (2019) The role of oxidative stress in the development of diabetes mellitus and its complications, J. Diabetes Res., 2019, 4189813, https://doi.org/10.1155/2019/4189813.
Varma, U., Koutsifeli, P., Benson, V. L., Mellor, K. M., and Delbridge, L. M. D. (2018) Molecular mechanisms of cardiac pathology in diabetes – experimental insights, Biochim. Biophys. Acta Mol. Basis Dis., 1864, 1949-1959, https://doi.org/10.1016/j.bbadis.2017.10.035.
Verma, S. K., Garikipati, V. N. S., and Kishore, R. (2017) Mitochondrial dysfunction and its impact on diabetic heart, Biochim. Biophys. Acta Mol. Basis Dis., 1863, 1098-1105, https://doi.org/10.1016/j.bbadis.2016.08.021.
Raza, H., Prabu, S.K., John, A., and Avadhani, N. G. (2011) Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats, Int. J. Mol. Sci., 12, 3133-3147, https://doi.org/10.3390/ijms12053133.
Ferreira, F.M., Palmeira, C. M., Seica, R., Moreno, A. J., and Santos, M. S. (2003) Diabetes and mitochondrial bioenergetics: alterations with age, J. Biochem. Mol. Toxicol., 17, 214-222, https://doi.org/10.1002/jbt.10081.
Marciniak, C., Marechal, X., Montaigne, D., Neviere, M., and Lancel, S. (2014) Cardiac contractile function and mitochondrial respiration in diabetes-related mouse models, Cardiovasc. Diabetol., 13, 118, https://doi.org/10.1186/s12933-014-0118-7.
Zhao, L., Dong, M., Xu, C., Zheng, H., Wei, T., et al. (2018) Identification of energy metabolism changes in diabetic cardiomyopathy rats using a metabonomic approach, Cell. Physiol. Biochem., 48, 934-946, https://doi.org/10.1159/000491960.
Lygate, C. A., and Neubauer, S. (2014) Metabolic flux as a predictor of heart failure prognosis, Circ. Res., 114, 1228-1230, https://doi.org/10.1161/CIRCRESAHA.114.303551.
Gataulin, R., Veselova, O., Studneva, I., Dobrokhotov, I., et al. (2021) Cardioprotective effects of galanin in a streptozotocin-induced diabetes mellitus rat model. 36th Annual Meeting of the International Society for Heart Research – European Section. Turin, Italy, Frontiers Event Abstracts, pp. 129-130, https://doi.org/10.3389/978-2-88971-002-7.
Zervou, S., Whittington, H. J., Russell, A. J., and Lygate, C. A. (2016) Augmentation of creatine in the heart, Mini Rev. Med. Chem., 16, 19-28, https://doi.org/10.2174/1389557515666150722102151.
Maritim, A. C., Sanders, R. A., and Watkins, J. B. (2003) Diabetes, oxidative stress and antioxidants. A Review, J. Biochem. Mol. Toxicol., 17, 24-38, https://doi.org/10.1002/jbt.10058.
Bayanes, J. W., and Thrope, S. R. (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm, Diabetes, 48, 1-9, https://doi.org/10.2337/diabetes.48.1.1.
Hall, R. L. (1991) Clinical pathology of laboratory animals, in Animal Models in Toxicology (Gad, S. C., and Chengelis, C. P., eds.) New York, Marcel Dekker Inc., pp. 765-811.
Huang, E., Kuo, W., Chen, Y., Chen, T., Chang, M., et al. (2006) Homocysteine and other biochemical parameters in type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy, Clin. Chim. Acta, 366, 293-298, https://doi.org/10.1016/j.cca.2005.10.025.
Fang, P., He, B., Yu, M., Shi, M., Zhu, Y., et al. (2018) Central galanin receptor 2 mediates galanin action to promote systemic glucose metabolism of type 2 diabetic rats, Biochem. Pharmacol., 156, 241-247, https://doi.org/10.1016/j.bcp.2018.08.036.
Legalkis, I. N., Mantzouridis, T., and Mountokalakis, T. (2007) Positive correlation of galanin with glucose in healthy volunteers during an oral glucose tolerance test, Horm. Metab. Res., 39, 53-55, https://doi.org/10.1055/s-2006-957346.
Gray, S., and Kim, J. K. (2011) New insights into insulin resistance in the diabetic heart, Trends Endocrinol. Metabol., 22, 394-403, https://doi.org/10.1016/j.tem.2011.05.001.
Tian, R., and Abel, E. D. (2001) Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis, Circulation, 103, 2961-2966, https://doi.org/10.1161/01.CIR.103.24.2961.
Wu, H., Deng, X., Shi, Y., Su, Ye, Wie, J., et al. (2016) PGC-1α, glucose metabolism and type 2 diabetes mellitus, J. Endocrinol., 229, R99-R115, https://doi.org/10.1530/JOE-16-0021.
Fang, P., Shib, M., Guo, L., He, B., Wang, Q., et al. (2014) Effect of endogenous galanin on glucose transporter 4 expression in cardiac muscle of type 2 diabetic rats, Peptides, 62, 159-163, https://doi.org/10.1016/j.peptides.2014.10.001.
Serebryakova, L., Studneva, I., Timoshin, A., Veselova, O., Pal’keeva, M., et al. (2021) Galanin peptides alleviate myocardial ischemia/reperfusion injury by reducing reactive oxygen species form, Inter. J. Peptide Res. Ther., 27, 2039-2048, https://doi.org/10.1007/s10989-021-10231-x.
Lang, R., Gundlach, A. L., Holmes, F. E., Hobson, S. A., Wynick, D., et al. (2015) Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity, Pharmacol. Rev., 67, 118-175, https://doi.org/10.1124/pr.112.006536.
Hausenloy, D. J., and Yellon, D. M. (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target, J. Clin. Invest., 123, 92-100, https://doi.org/10.1172/JCI62874.
Jay, M. A., and Ren, J. (2007) Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus, Curr. Diab. Rev., 3, 33-39, https://doi.org/10.2174/157339907779802067.
Funding
The work was supported by the Russian Foundation for Basic Research (projects nos. 18-015-0008-a and 18-015-0009-a) and Ministry of Health of the Russian Federation (registration no. NIOKTR 121031700143-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interests. The study is performed in compliance with the international and/or institutional principles of care and use of animals.
Rights and permissions
About this article
Cite this article
Studneva, I.M., Veselova, O.M., Dobrokhotov, I.V. et al. Chimeric Agonist of Galanin Receptor GALR2 Reduces Heart Damage in Rats with Streptozotocin-Induced Diabetes. Biochemistry Moscow 87, 346–355 (2022). https://doi.org/10.1134/S0006297922040046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922040046