Abstract
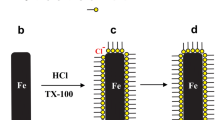
The inhibiting action of nonionic surfactants, namely, propoxylated fatty esters (PFE) with different number of propylene oxide toward the corrosion of Zn in 0.1 M HCl solutions was investigated. Four techniques were used in this study such as weight loss, galvanostatic polarization, potentiodynamic anodic polarization and electrochemical impedance spectroscopy. The inhibition efficiency of PFE compounds increases by increasing the concentrations of PFE, number of propylene oxide unit and with lowering temperature. The inhibition was interpreted by adsorption of PFE molecules on the Zn surface according Temkin’s isotherm. Galvanstatic polarization indicated that the PFE acts as a mixed type inhibitor. PFE molecules inhibit the pitting corrosion of Zn in a solution containing Cl– ions by moving the pitting potentials into a more noble direction. The activation thermodynamic parameters such as \(E_{{\text{a}}}^{*}\), ΔH* and ΔS* are calculated and explained.









Similar content being viewed by others
REFERENCES
Shah, M.D., Patel, A.S., Mudaliar, G.V., and Shah, N.K., Port. Electrochim. Acta, 2011, vol. 29, p. 10.
Abdallah, M., Corros. Sci., 2003, vol. 45, p. 2705.
Agrawal, Y.K., Talati, J.D., Desai, M.N., and Shah, N.K., Corros. Sci., 2004, vol. 46, p. 633.
Abdallah, M., El-Etre, A.Y., and Moustafa, M.F., Port. Electrochim. Acta, 2009, vol. 27, p. 615.
Fouda, A.S., Abdallah, M., Atwa, S.T., and Salem, M.M., Mod. Appl. Sci., 2010, vol. 4, p. 41.
Abdallah, M., Zaafarany, I., Fouda, A.S., and Abd El-Kader, D., J. Mater. Eng. Perform., 2012, vol. 21, p. 995.
Saadawy, M., Int. J. Electrochem. Sci., 2018, vol. 13, pp. 9600–9617.
Abdallah, M., Atwa, S.T., Salem, M.M., and Fouda, A.S., Int. J. Electrochem. Sci., 2013, vol. 8. p. 10001.
Wang, L., Pu, J.X., and Luo, H., Corros. Sci., 2002, vol. 45, p. 677.
Fouda, A.S., Rashwan, S., Emam, A., and El-Morsy, F.E., Int. J. Electrochem. Sci., 2018, vol. 13, p. 3719.
Abdallah, M., Zaafarany, I.A., Fouda, A.S., and El-Nagar, W., J. Electrochem. Plat. Technol., 2014, vol. 2, p. 2.
Popoola, A.P.I. and Fayomi, O.S.I., Int. J. Electrochem. Sci., 2011, vol. 6, p. 4581.
Abdallah, M., Zaafarany, I.A., and AL Jahdaly, B.A., J.Mater. Environ. Sci., 2016, vol. 7, p. 1107.
Yousif, E., Win, Y., Al-Hamadani, A.H., Al-Amiery, A.A., Kadhum, A.A., and Mohamad, A., Int. J. Electrochem. Sci., 2015, vol. 10, p. 1708.
M. Abdallah, Elshafie A. M. Gad, Jabir H Al-Fahemi, and M. Sobhi // Protection of Metals and Physical Chemistry of Surfaces, 2018, Vol. 54, No. 3, pp. 503–512. https://doi.org/10.1134/S207020511803022X
Abdallah, M., Hazazi, O.A., Fouda, A.S., and Abdel-Fatah, A., Prot. Met. Phys. Chem. Surf., 2015, vol. 51, p. 473.
Abdallah, M., Hegazy, M.A., Alfakeer, M., and Ahmed, H., Green Chem. Lett. Rev., 2018, vol. 11, p. 457.
Fawzy, A., Zaafarany, I.A., Ali, H.M., and Abdallah, M., Int. J. Electrochem. Sci., 2018, vol. 13, p. 4575.
Fawzy, A., Abdallah, M., Zaafarany, I.A., Ahmed, S.A., and Althagafi, I.I., J. Mol. Liq., 2018, vol. 265, p. 276.
Abdallah, M., Al Jahdaly, B.A., and Al-Malyo, O.A., Int. J. Electrochem. Sci., 2015, vol. 10, p. 2740.
Abdallah, M., Al Jahdaly, B.A., Sobhi, M., and Ali, A.I., Int. J. Electrochem. Sci., 2015, vol. 10, p. 4482.
Hegazy, M.A., Abdallah, M., Alfakeer, M., and Ahmed, H., Int. J. Electrochem. Sci., 2018, vol. 13, p. 6824.
Sobhi, M., El-Sayed, R., and Abdallah, M., J. Surfactants Deterg., 2013, vol. 16, p. 937.
Sobhi, M., El-Sayed, R., and Abdallah, M., Chem. Eng. Commun., 2016, vol. 203, p. 758.
Mathur, P.B. and Vasudevan, T., Corrosion, 1982, vol. 38, p. 17.
Hazazi, O.A. and Abdallah, M., Int. J. Electrochem. Sci., 2013, vol. 8, p. 8138.
Graciaa, A., Barakat, Y., Schechter, R.S., Wade, W.H., and Yiv S., J. Colloid Interface Sci., 1982, vol. 89, p. 219.
Sobhi, M., Abdallah, M., and Khairou, K.S., Monatsh. Chem., 2012, vol. 143, p. 1379.
Laidler, K.J., Chemical Kinetics, New York: McGraw-Hill, 1965.
Abdallah, M., Salem, M.M., AL Jahdaly, B.A., Awad-E. Helal, M.I., and Fouda, A.S., Int. J. Electrochem. Sci., 2017, vol. 12. p. 4543.
Abdallah, M., Fouda, A.S., El-Nagar, D.A.M., and Ghoneim, M.M., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, p. 1204.
Bayol, E., Kayakirilmaz, K., and Erbil, M., Mater. Chem. Phys., 2007, vol. 104, p. 74.
Benali, O., Larabi, L., Traisnel, M., Gengembre, L., and Harek, Y., Appl. Surf. Sci., 2007, vol. 253, p. 6130.
Abdallah, M., Al-Karane, S.A., and Fattah, A.A.A., Prot. Met. Phys. Chem. Surf., 2009, vol. 50, p. 205.
Hameed, R.S.A. and Abdallah, M., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, p. 113.
Abdallah, M., Altass, H.M., AL-Jahdaly, B.A., and Salem, M.M., Green Chem. Lett. Rev., 2018, vol. 11, p. 189.
Abdallah, M., AL-Jahdaly, B.A., Salem, M.M., Fawzy, A., and Fattah, A.A.A., J.Mater. Environ. Sci., 2017, vol. 8, p. 2599.
Putilova, I., Balezin, S., and Barannik, V., Metallic Corrosion Inhibitors, Oxford: Pergamon, 1960.
Temkin, M.I., Zh. Fiz. Khim., 1941, vol. 15, p. 296.
Fouda, A.S., Abdallah, M., Al-Ashry, S.M., and Fattah, A.A.A., Desalination, 2010, vol. 250, p. 538.
Tedeschi, X.R.J., Corrosion, 1975, vol. 31, p.130.
Zaafarany, I. and Abdallah, M., Int. J. Electrochem. Sci., 2010, vol. 5, p. 18.
Funding
This research was funded by the Environmental Pollution Research Chair at Princes Noura bint Abdulrahman University (grant no. Ep-06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alfakeer, M., Abdallah, M. & Abdel Hameed, R.S. Propoxylated Fatty Esters as Safe Inhibitors for Corrosion of Zinc in Hydrochloric Acid. Prot Met Phys Chem Surf 56, 225–232 (2020). https://doi.org/10.1134/S2070205120010025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205120010025