Abstract
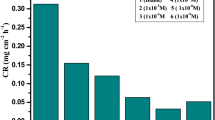
Electro-oxidation and inhibitor performance of copper specimens in 1 M hydrochloric acid solution was investigated at room temperature by linear potentiodynamic polarization and gravimetric method in the presence of 2-aminoethanol (A) and N, N-diethylethanamine (D) as an inorganic inhibitor. The effect of the inhibitory concentration on the corrosion behavior of copper was studied over 288 hrs at 298°K. The inhibitory efficiency rise up to 96% for single induced and 98% for synergistic behavior. The adsorption mechanism characteristic was supported by SEM/EDX analysis and adsorption isotherm. From all indication, the inhibitive efficiency of these compounds majorly depends on their molecular structure and concentration. The blocking effects of the surface interface were also explained on the basis of the inhibitor active action. 2-aminoethanol and N, N-diethylethanamine inhibits copper in 1 M HCl by strictly affecting both the anodic and cathodic sites. Portion of the surface covered calculated was also found to follow Langmuir adsorption isotherm.
Similar content being viewed by others
References
Antonijevic, M.M. and Petrovic, M.B., Int. J. Electrochem. Sci., 2012, vol. 3, p. 1.
Trachli, B., Keddam, M., Takenouti, H., and Srhiri, A., Science, 2002, vol. 44, p. 997.
Deslouis, C., Tribollet, B., Mengoli, G., and Musiani, M.M., J. Appl. Electrochem., 1988, vol. 18, p. 374.
Shim, J.J. and Kim, J.G., Mater. Lett., 2004, vol. 58, p. 2002.
Mountassir, Z. and Srhiri, A., Corros. Sci., 2007, vol. 49, p. 1350.
Reitmeier, R.E, Sivertz, V., and Tartar, H.V., J. Am. Chem. Soc., 1940, vol. 62, p. 1943.
Otmacic, H., Telegdi, J., Papp K., and StupnisekLisac, E., J. Appl. Electrochem., 2004, vol. 34, p. 545.
Bartley, J., Huynh, N., Bottle, S.E., et al., Corros. Sci., 2003, vol. 45, p. 81.
Abdulwahab, M, Popoola, A.P.I., and Fayomi, O.S.I., Int. J. Electrochem. Sci., 2012, vol. 7, p. 11706.
Libralato, G., Volpi Ghirardini, A., and Avezzù, F., J. Hazard Mater., 2009, vol. 176, p. 535.
Rodrigues, P.R.P., Aoki, I.V., De Andrade, A.H.P., et al., Br. Corros. J., 1996, vol. 31, p. 305.
Frignani, A., Tommesani, L., Brunoro, G., et al., Corros. Sci., 1999, vol. 41, p. 1205.
Rodrigues, P.R.P., Zerbino, J.O., and Agostinho, S.M.L., Mater. Sci. Forum., 1998, vol. 289, p. 1299.
Popoola, A.P.I, Abdulwahab, M., and Fayomi, O.S.J., Int. J. Electrochem. Sci., 2012, vol. 7, p. 5805.
Bentiss, F., Bouanis, M., Mernari, B., et al., Appl. Surf. Sci., 2007, vol. 253, p. 3696.
Li, W., He, Q., Pei, C., and Hou, B., Electrochem. Acta, 2007, vol. 52, p. 6386.
Wang, L., Corros. Sci., 2006, vol. 48, p. 608.
Kosec, T., Milošev I., and Pihlar B., Appl. Surf. Sci., 2007, vol. 253, p. 8863.
Otieno-Alego, V., Hope, G.A., Notoya, T., and Schweinsberg, D.P., Corros. Sci., 1996, vol. 38, p. 213.
Schweinsberg, D.P., Bottle, S.E., Otieno-Alego, V., and Notoya, T., J. Appl. Electrochem., 1997, vol. 27, p. 161.
Ma, H., Chen, S., Niu, L., et al., J. Appl. Electrochem., 2002, vol. 32, p. 65.
Abdulwahab, M., Kasim, A., Fayomi, O.S.I., et al., J. Mater. Environ. Sci., 2012, vol. 3, p. 1177.
Ehteram, A.N. and Aisha, H.A., Int. J. Electrochem. Sci., 2008, vol. 98, p. 806.
Fayomi, O.S.I. and Popoola, A.P.I., Res. J. Chem Environ., 2008, vol. 17, p. 99.
Satpati, A.K. and Ravindran, P.V., Mat. Chem. Phys., 2008, vol. 109, p. 352.
Fayomi, O.S.I., Popoola, A.P.I., Abdulwahab, M., and Popoola, O.M., Int. J. Res. Eng. Soc. Sci., 2012, vol. 2, p. 13.
Mountassir, Z. and Srhiri, A., Corros. Sci., 2007, vol. 49, p. 1350.
Itagaki, M., Tagaki, M., and Watanabe, K., Corros. Sci., 1996, vol. 38, p. 1109.
Tromans, D. and Silva, J.C., Corros. Sci., 1997, vol. 53, p. 171.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Fayomi, O.S.I., Popoola, A.P.I. Electrochemical oxidation assessment and interaction of 2-aminoethanol and N, N-diethylethanamine propagation in acidic medium. Prot Met Phys Chem Surf 51, 891–898 (2015). https://doi.org/10.1134/S2070205115050081
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205115050081