Abstract
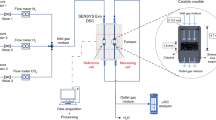
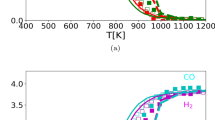
The most correct method for studying the peculiarities of stationary kinetics of heterogeneous reactions is the flowing circulation method which has been substantially improved in recent years. The core of the introduced improvement lies in the direct determination of reaction velocity (catalytic activeness) at the given compound of the contact reaction mixture. The improved flowing circulation method was applied for the first time to study the deep oxidation of methane by molecular oxygen in order to obtain one-parameter dependences of catalytic reactivity on the temperature and concentration of reaction medium components and the solution of the inverse kinetic problem. Catalyst IK 12–72 compound, wt %: 16 ± 2 Cr2O3, 4 ± 0.6 MgO and the remaining Al2O3 has been studied. In the kinetic area, the apparent energy of reaction activation (110 kJ/mol) has been determined. On the basis of experiments and ideas on the mechanism of the Langmuir-Hinshelwood reaction of competitive absorption type, the equation well describing experimental data in the overall studied range of concentrations (average relative deviation 8%) was selected. The simplicity of the improved flowing circulation method allows for it to be recommended for the selection of the most active catalysts and analysis of dependences of stationary velocities of heterogeneous catalytic reactions on various process parameters.
Similar content being viewed by others
References
USSR Inventor Certificate No. 1216862, 6-09-5505-88.
Parmon, V.N., Ismagilov, Z.R., Favorskii, O.N., et al., Vestn. Ross. Akad. Nauk, 2007, vol. 77, no. 9, p. 819.
Temkin, M.I., Kiperman, S.L., and Lukiyanova, L.I., Dokl. Akad. Nauk SSSR, 1950, vol. LXXIY, no. 4, p. 763.
Bobrov N.N., Parmon V.N., in Rapid catalyst testing in the Boreskov Institute of Catalysis, NATO Sci. Ser. II: Math., Phys. Chem. 2002, vol. 69, p. 197.
Bobrov, N.N., RF Patent 2085938.
Bobrov, N.N., RF Patent 2162366.
Bobrov, N.N., Industrial Catalysis in Lectures, issue 3, Moscow: Kalvis, 2006, p. 41.
Bobrov, N.N., Leonov, A.S., Belov, A.N., et al., Catalysis in the Industry, 2005, issue 2, p. 50.
Samokhov, A.A., Materials of the Coordination Center, Novosibirsk, IK SO RAN, 1973, no. 4, p. 3.
Andrushkevich, T.V., Popovskiy, V.V., Boreskov, G.K., Kinet. Katal., 1965, vol. 6, no. 5, p. 860.
Ermakova, A., Industrial Catalysis in Lectures, issue 4, Moscow, Kalvis, 2006.
Author information
Authors and Affiliations
Additional information
Original Russian Text © I.Yu. Pakharukov, N.N. Bobrov, V.N. Parmon, 2009, published in Kataliz v Promyshlennosti.
Rights and permissions
About this article
Cite this article
Pakharukov, I.Y., Bobrov, N.N. & Parmon, V.N. Investigation of deep methane oxidation with the use of the improved flowing circulation method. Catal. Ind. 1, 38–42 (2009). https://doi.org/10.1134/S207005040901005X
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207005040901005X