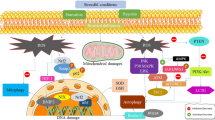
Abstract—
Neurodegeneration is a complex progressive pathological process leading to the neuronal death, which is induced by various external and internal factors. Neurodegenerative diseases, injuries of the central and peripheral nervous system, mental disorders, and a number of other pathological conditions, accompanied by functional and structural degradation of neurons and their death, is a serious problem in the global healthcare system, as due to these diseases millions of people around the world become disabled or die every year. The situation is complicated by the lack of selective, clinically effective neuroprotective drugs. It has been shown that nitric oxide (NO) and hydrogen sulfide (H2S) are actively involved in neurodegeneration and cell death of neurons and glia, but their role is not completely clear. This review considers NO- and H2S-dependent signaling mechanisms underlying the pathogenesis of neurodegenerative processes. The prospects for further studies of the role of NO and H2S in the nervous tissue under conditions of pathological conditions associated with neurodegeneration are considered.








Similar content being viewed by others
REFERENCES
Przedborski S., Vila M., Jackson-Lewis V. 2003. Series introduction neurodegeneration what is it and where are we? J. Clin. Invest. 111, 3–10.
Pérez-Neri I., Ramírez-Bermúdez J., Montes S., Ríos C. 2006. Possible mechanisms of neurodegeneration in schizophrenia. Neurochem. Res. 31, 1279–1294.
Dzreyan V., Rodkin S., Nikul V., Pitinova M., Uzdensky A. 2021. The expression of E2F1, p53, and caspase 3 in the rat dorsal root ganglia after sciatic nerve transection. J. Mol. Neurosci. 71, 826–835.
Dreßler J., Hanisch U., Kuhlisch E., Geiger K. 2007. Neuronal and glial apoptosis in human traumatic brain injury. Int. J. Legal Med. 121, 365–375.
Rodkin S.V., Dzreyan V.A., Demyanenko S.V., Uzdensky V.B. 2021. The role of p53-dependent signaling pathways in survival and death of neurons and glial cells in peripheral nerve injury. Biochemistry (Moscow), Suppl. Series A: Membr. Cell Biol. 15, 334–347.
Flores G., Morales-Medina J., Diaz A. 2016. Neuronal and brain morphological changes in animal models of schizophrenia. Behav. Brain Res. 301, 190–203.
Nakamura T., Lipton S. 2017. ‘SNO’-storms compromise protein activity and mitochondrial metabolism in neurodegenerative disorders. Trends Endocrinol. Metab. 28, 879–892.
Muchowski P., Wacker J. 2005. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 6, 11–22.
Giovinazzo D., Bursac B., Sbodio J., Nalluru S., Vignane T., Snowman A., Albacarys L., Sedlak T., Torregrossa R., Whiteman M., Filipovic M., Snyder S., Paul D. 2021. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA. 118, e2017225118.
Stewart V., Heales S. 2003. Nitric oxide-induced mitochondrial dysfunction implications for neurodegeneration. Free Radic. Biol. Med. 34, 287–303.
Chen K., Northington F., Martin L. 2010. Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct. Funct. 214, 219–234.
Jung J., Jeong N. 2014. Hydrogen sulfide controls peripheral nerve degeneration and regeneration a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases. Neural. Regen. Res. 9, 2119.
Caviedes A., Varas-Godoy M., Lafourcade C., Sandoval S., Bravo-Alegria J., Kaehne T., Massmann A., Figueroa J., Nualart F., Wyneken U. 2017. Endothelial nitric oxide synthase is present in dendritic spines of neurons in primary cultures. Front. Cell. Neurosci. 11, 180.
Lin Y., Liang H., Xu K., Ni H., Dong J., Xiao H., Chang L., Wu H., Li F., Zhu D., Luo C. 2018. Dissociation of nNOS from PSD-95 promotes functional recovery after cerebral ischaemia in mice through reducing excessive tonic GABA release from reactive astrocytes. J. Pathol. 244, 176–188.
Hou X., Hu Z., Zhang D., Lu W., Zhou J., Wu P., Guan X., Han Q., Deng S., Zhang H., Chen J., Wang F. 2017. Rapid antidepressant effect of hydrogen sulfide evidence for activation of mTORC1-TrkB-AMPA receptor pathways. Antioxid. Redox Signal. 27, 472–488.
Wang Y., Hong F., Yang S. 2022. Roles of nitric oxide in brain ischemia and reperfusion. Int. J. Mol. Sci. 23, 4243.
Ghanbari F., Khaksari M., Vaezi G., Hojati V., Shiravi A. 2019. Hydrogen sulfide protects hippocampal neurons against methamphetamine neurotoxicity via inhibition of apoptosis and neuroinflammation. J. Mol. Neurosci. 67, 133–141.
Park K., Lee Y., Park S., Lee S., Hong Y., Kil S., Hong Y. 2010. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J. Pineal Res. 48, 270–281.
Zhang J., Zhang S., Shan H., Zhang M. 2020. Biologic effect of hydrogen sulfide and its role in traumatic brain injury. Oxid. Med. Cell. Longev. 2020, 7301615.
Qu W., Cheng Y., Peng W., Wu Y., Rui T., Luo C., Zhang J. 2022. Targeting iNOS alleviates early brain injury after experimental subarachnoid hemorrhage via promoting ferroptosis of M1 microglia and reducing neuroinflammation. Mol. Neurobiol. 59, 3124–3139.
Jin R., Yang R., Cui C., Zhang H., Cai J., Geng B., Chen Z. 2022. Ferroptosis due to cystathionine γ lyase/hydrogen sulfide downregulation under high hydrostatic pressure exacerbates VSMC dysfunction. Front. Cell Dev. Biol. 10, 829316.
Xu X., Shi R., Fu Y., Wang J., Tong X., Zhang S., Wang N., Li M., Tong Y., Wang W., He M., Liu B., Chen G., Guo F. 2023. Neuronal nitric oxide synthase/reactive oxygen species pathway is involved in apoptosis and pyroptosis in epilepsy. Neural Regen. Res. 18, 1277.
Yang K., Li W., Liu Y., Wei Y., Ren Y., Mai C., Zhang S., Zuo Y., Sun Z., Li D., Yang C. 2022. Hydrogen sulfide attenuates neuroinflammation by inhibiting the NLRP3/Caspase-1/GSDMD pathway in retina or brain neuron following rat ischemia/reperfusion. Brain Sci. 12, 1245.
Chen Y., Chen Y., Chiu H., Ko Y., Wang R., Wang W., Chuang Y., Huang C., Lu T. 2021. Cell-penetrating delivery of nitric oxide by biocompatible dinitrosyl iron complex and its dermato-physiological implications. Int. J. Mol. Sci. 22, 10101.
Goshi E., Zhou G., He Q. 2019. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 9, 192–207.
Hall C., Garthwaite J. 2009. What is the real physiological NO concentration in vivo? Nitric Oxide. 21, 92–103.
Forstermann U., Sessa W. 2012. Nitric oxide synthases regulation and function. Eur. Heart J. 33, 829–837.
Lvova O.A., Orlova A.E., Gusev V.V., Kovtun O.P., Chegodaev D.A. 2010. To the question of the role of nitric oxide in norm and pathology of the nervous system. System Integral Healthcare. 4, 20–35.
Kone B. 2001. Molecular biology of natriuretic peptides and nitric oxide synthases. Cardiovasc. Res. 51, 429–441.
Gusakova S.V., Kovalev I.V., Smaglii L.V., Birulina Y.G., Nosarev A.V., Petrova I.V., Medvedev M.A., Orlov S.N., Reutov V.P. 2015. Gas signaling in mammalian cells. Uspekhi fiziol. nauk. (Rus.). 46, 53–73.
Keszler A., Lindemer B., Hogg N., Weihrauch D., Lohr N. 2018. Wavelength-dependence of vasodilation and NO release from S-nitrosothiols and dinitrosyl iron complexes by far red/near infrared light. Arch. Biochem. Biophys. 649, 47–52.
Vincent S. 2010. Nitric oxide neurons and neurotransmission. Prog. Neurobiol. 90, 246–255.
Yang G., Chen G., Ebner T., Iadecola C. 1999. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am. J. Physiol. Integr. Comp. Physiol. 277, R1760–R1770.
Kodama T., Koyama Y. 2006. Nitric oxide from the laterodorsal tegmental neurons its possible retrograde modulation on norepinephrine release from the axon terminal of the locus coeruleus neurons. Neuroscience. 138, 245–256.
Vincent S. 2000. The ascending reticular activating system – from aminergic neurons to nitric oxide. J. Chem. Neuroanat. 18, 23–30.
Lin D., Fretier P., Jiang C., Vincent S. 2010. Nitric oxide signaling via cGMP-stimulated phosphodiesterase in striatal neurons. Synapse. 64, 460–466.
Yakovleva O.V., Shafigullin M.U., Sitdikova G.F. 2013. The role of nitric oxide in the regulation of mediator secretion and processes of exo- and endocytosis of synaptic vesicles in mouse motor nerve endings. Neurochem J. 7, 103–110.
Garthwaite J. 2019. NO as a multimodal transmitter in the brain discovery and current status. Br. J. Pharmacol. 176, 197–211.
Gallo E., Iadecola C. 2011. Neuronal nitric oxide contributes to neuroplasticity-associated protein expression through cGMP protein kinase g and extracellular signal-regulated kinase. J. Neurosci. 31, 6947–6955.
Bradley S., Steinert J. 2016. Nitric oxide-mediated posttranslational modifications impacts at the synapse. Oxid. Med. Cell. Longev. 2016, 5681036.
Selvakumar B., Jenkins M., Hussain N., Huganir R., Traynelis S., Snyder S. 2013. S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation single-channel conductance and endocytosis. Proc. Natl. Acad. Sci. USA. 110,1077–1082.
Schafe G., Bauer E., Rosis S., Farb C., Rodrigues S., LeDoux J. 2005. Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. Eur. J. Neurosci. 22, 201–211.
Sudorgina P.V., Saulskaya N.B. 2015. Sound danger signals activate the nitrergic system of the medial prefrontal cortex. Neurosci. Behav. Physiol. 46, 1017–1023.
Balaban P.M., Roshchin M.V., Korshunova T.A. 2011. Two-faced nitric oxide is necessary for both memory erasure and memory formation. Neurosci. Behav. Physiol. 42, 895–900.
Ill-Raga G., Tajes M., Busquets-García A., Ramos-Fernández E., Vargas L., Bosch-Morató M., Guivernau B., Valls-Comamala V., Eraso-Pichot A., Guix F., Fandos C., Rosen M., Rabinowitz M., Maldonado R., Alvarez A., Ozaita A., Muñoz F. 2015. Physiological control of nitric oxide in neuronal BACE1 translation by heme-regulated eIF2α kinase HRI induces synaptogenesis. Antioxid. Redox Signal. 22, 1295–1307.
Ill-Raga G., Köhler C., Radiske A., Lima R., Rosen M., Muñoz F., Cammarota M. 2013. Consolidation of object recognition memory requires HRI kinase-dependent phosphorylation of eIF2α in the hippocampus. Hippocampus. 23, 431–436.
Bel E., Del Guimarães F., Bermũdez-Echeverry M., Gomes M., Schiaveto-de-Souza A., Padovan-Neto F., Tumas V., Barion-Cavalcanti A., Lazzarini M., Nucci-da-Silva L., de Paula-Souza D. 2005. Role of nitric oxide on motor behavior. Cell. Mol. Neurobiol. 25, 371–392.
Contestabile A. 2012. Role of nitric oxide in cerebellar development and function focus on granule neurons. Cerebellum. 11, 50–61.
Xiao Q., Ying J., Xiang L., Zhang C. 2018. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine (Baltimore). 97, e13065.
Kolesnikov S., Vlasov B., Kolesnikova L. 2015. Hydrogen as a third essential gas molecule in living tissues. Ann. Russ. Acad. Med. Sci. 70, 237–241.
Lu D., Wang L., Liu G., Wang S., Wang Y., Wu Y., Wang J., Sun X. 2022. Role of hydrogen sulfide in subarachnoid hemorrhage. CNS Neurosci. Ther. 28, 805–817.
Kimura H., Shibuya N., Kimura Y. 2012. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid. Redox Signal. 17, 45–57.
Furne J., Saeed A., Levitt M.D. 2008. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1479–R1485.
Rodkin S., Nwosu C., Sannikov A., Tyurin A., Chulkov V., Raevskaya M., Ermakov A., Kirichenko E., Gasanov M. 2023. The role of gasotransmitter-dependent signaling mechanisms in apoptotic cell death in cardiovascular rheumatic kidney and neurodegenerative diseases and mental disorders. Int. J. Mol. Sci. 24, 6014.
Sen N. 2017. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J. Mol. Biol. 429, 543–561.
Zhu H., Blake S., Chan K., Pearson R., Kang J. 2018. Cystathionine β -synthase in physiology and cancer. Biomed. Res. Int. 11, 3205125.
Zuhra K., Augsburger F., Majtan T., Szabo C. 2020. Cystathionine-β-synthase molecular regulation and pharmacological inhibition. Biomolecules. 10, 697.
Jurkowska H., Kaczor-Kamińska M., Bronowicka-Adamska P., Wróbel M. 2014. Cystathionine γ-lyase. Postepy Hig. Med. Dosw. 68, 1–9.
Sharif A., Iqbal M., Manhoosh B., Gholampoor N., Ma D., Marwah M., Sanchez-Aranguren L. 2023. Hydrogen sulphide-based therapeutics for neurological conditions perspectives and challenges. Neurochem. Res. 48, 1981–1996
Lupoli R., Di Minno A., Spadarella G., Franchini M., Sorrentino R., Cirino G., Di Minno G. 2015. Methylation reactions the redox balance and atherothrombosis the search for a link with hydrogen sulfide. Semin. Thromb. Hemost. 41, 423–432.
Yakovlev A., Kurmasheva E., Ishchenko Y., Giniatullin R., Sitdikova G. 2017. Age-dependent subunit specific action of hydrogen sulfide on GluN1/2A and GluN1/2B NMDA receptors. Front. Cell. Neurosci. 11, 375.
Munaron L., Avanzato D., Moccia F., Mancardi D. 2013. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 53, 77–84.
Liu D., Wang Z., Zhan J., Zhang Q., Wang J., Zhang Q., Xian X., Luan Q., Hao A. 2014. Hydrogen sulfide promotes proliferation and neuronal differentiation of neural stem cells and protects hypoxia-induced decrease in hippocampal neurogenesis. Pharmacol. Biochem. Behav. 116, 55–63.
Kamat P., Kalani A. 2015. Abstract T P87 hydrogen sulfide enhances neurogenesis through the IRAK-1/GSK3β/AKT signaling pathways after ischemic stroke. Stroke. 46, ATP87-ATP87.
Pomierny B., Krzyżanowska W., Jurczyk J., Skórkowska A., Strach B., Szafarz M., Przejczowska-Pomierny K., Torregrossa R., Whiteman M., Marcinkowska M., Pera J., Budziszewska B. 2021.The slow-releasing and mitochondria-targeted hydrogen sulfide (H2S) Delivery molecule AP39 induces brain tolerance to ischemia. Int. J. Mol. Sci. 22, 7816.
Mohseni F., Bagheri F., Rafaiee R., Norozi P., Khaksari M. 2020. Hydrogen sulfide improves spatial memory impairment via increases of BDNF expression and hippocampal neurogenesis following early postnatal alcohol exposure. Physiol. Behav. 215, 112784.
Varaksin A.A., Pushchina E.V. 2012. Significance of hydrogen sulfide in the regulation of organ functions. Tikhookeanskii med. zhurnal. (Rus.). 2, 27–36.
Yakovlev A.V., Sitdikova G.F. 2014. Physiological role of hydrogen sulfide in the nervous system. Genes & Cells. 9, 34–40.
Wang M., Zhu J., Pan Y., Dong J., Zhang L., Zhang X., Zhang L. 2015. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson’s disease. J. Neurosci. Res. 93, 487–494.
Ali R., Pal H., Hameed R., Nazir A., Verma S. 2019. Controlled release of hydrogen sulfide significantly reduces ROS stress and increases dopamine levels in transgenic C. elegans. Chem. Commun. 55, 10142–10145.
Sitdikova G.F., Yakovlev A.V., Odnoshivkina Y.G., Zefirov A.L. 2011. Effect of hydrogen sulfide on the processes of exo- and endocytosis of synaptic vesicles in the frog motor nerve ending. Neurochem. J. 28, 280–286.
Gerasimova E., Lebedeva J., Yakovlev A., Zefirov A., Giniatullin R., Sitdikova G. 2015. Mechanisms of hydrogen sulfide (H2S) action on synaptic transmission at the mouse neuromuscular junction. Neuroscience. 303, 577–585.
Wang Y., Jia J., Ao G., Hu L., Liu H., Xiao Y., Du H., Alkayed N., Liu C., Cheng J. 2014. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J. Neurochem. 129, 827–838.
Campolo M., Esposito E., Ahmad A., Di Paola R., Paterniti I., Cordaro M., Bruschetta G., Wallace J., Cuzzocrea S. 2014. Hydrogen sulfide-releasing cyclooxygenase inhibitor ATB-346 enhances motor function and reduces cortical lesion volume following traumatic brain injury in mice. J. Neuroinflammation. 11, 196.
Tabassum R., Jeong N. 2019. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 16, 1386–1396.
Zhang M., Shan H., Wang T., Liu W., Wang Y., Wang L., Zhang L., Chang P., Dong W., Chen X., Tao L. 2013. Dynamic change of hydrogen sulfide after traumatic brain injury and its effect in mice. Neurochem. Res. 38, 714–725.
Ide M., Ohnishi T., Toyoshima M., Balan S., Maekawa M., Shimamoto-Mitsuyama C., Iwayama Y., Ohba H., Watanabe A., Ishii T., Shibuya N., Kimura Y., Hisano Y., Murata Y., Hara T., Morikawa M., Hashimoto K., Nozaki Y., Toyota T., Wada Y., Yoshikawa T. 2019. Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Mol. Med. 11, e10695.
Gopalakrishnan P., Shrestha B., Kaskas A., Green J., Alexander J., Pattillo C. 2019. Hydrogen sulfide therapeutic or injurious in ischemic stroke? Pathophysiology. 26, 1–10.
DiSabato D., Quan N., Godbout J. 2016. Neuroinflammation: The devil is in the details. J. Neurochem. 139, 136–153.
Woodburn S., Bollinger J., Wohleb E. 2021. The semantics of microglia activation neuroinflammation homeostasis and stress. J. Neuroinflammation. 18, 258.
Liao R., Wood T., Nance E. 2020. Nanotherapeutic modulation of excitotoxicity and oxidative stress in acute brain injury. Nanobiomedicine. 7, 184954352097081.
Hirsch E., Vyas S., Hunot S. 2012. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 18, S210–S212.
Kummer M., Hermes M., Delekarte A., Hammerschmidt T., Kumar S., Terwel D., Walter J., Pape H., König S., Roeber S., Jessen F., Klockgether T., Korte M., Heneka M. 2011. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron. 71, 833–844.
Evonuk K., Doyle R., Moseley C., Thornell I., Adler K., Bingaman A., Bevensee M., Weaver C., Min B., DeSilva T. 2020. Reduction of AMPA receptor activity on mature oligodendrocytes attenuates loss of myelinated axons in autoimmune neuroinflammation. Sci. Adv. 6, eaax5936.
Alawieh A., Langley E., Weber S., Adkins D., Tomlinson S. 2018. Identifying the role of complement in triggering neuroinflammation after traumatic brain injury. J. Neurosci. 38, 2519–2532.
Picca A., Calvani R., Coelho-Junior H., Landi F., Bernabei R., Marzetti E. 2020. Mitochondrial dysfunction oxidative stress and neuroinflammation intertwined roads to neurodegeneration. Antioxidants. 9, 647.
Gao F., Lucke-Wold B., Li X., Logsdon A., Xu L., Xu S., LaPenna K., Wang H., Talukder M., Siedlecki C., Huber J., Rosen C., He P. 2018. Reduction of endothelial nitric oxide increases the adhesiveness of constitutive endothelial membrane ICAM-1 through src-mediated phosphorylation. Front. Physiol. 8, 1124.
Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C., Buerk D., Huang P., Jain R. 2001. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. 98, 2604–2609.
Hollenberg S., Guglielmi M., Parrillo J. 2007. Discordance between microvascular permeability and leukocyte dynamics in septic inducible nitric oxide synthase deficient mice. Crit. Care. 11, R125.
Chen Z., Mou R., Feng D., Wang Z., Chen G. 2017. The role of nitric oxide in stroke. Med. Gas Res. 7, 194.
Oh G., Pae H., Lee B., Kim B., Kim J., Kim H., Jeon S., Jeon W., Chae H., Chung H. 2006. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 41, 106–119.
Kondo K., Bhushan S., King A., Prabhu S., Hamid T., Koenig S., Murohara T., Predmore B., Gojon G., Gojon G., Wang R., Karusula N., Nicholson C., Calvert J., Lefer D. 2013. H2S protects against pressure overload–induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 127, 1116–1127.
Liy P., Puzi N., Jose S., Vidyadaran S. 2021. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 246, 2399–2406.
Pandareesh M., Anand T. 2014. Neuroprotective and anti-apoptotic propensity of bacopa monniera extract against sodium nitroprusside induced activation of inos heat shock proteins and apoptotic markers in PC12 cells. Neurochem. Res. 39, 800–814.
Radi E., Formichi P., Battisti C., Federico A. 2014. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 42, S125–S152.
Xie Z., Liu Y., Bian J. 2016. Hydrogen sulfide and cellular redox homeostasis. Oxid. Med. Cell. Longev. 2016, 6043038.
Corsello T., Komaravelli N., Casola A. 2018. Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants. 7, 129.
Bruce King S. 2013. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic. Biol. Med. 55, 1–7.
Austin S., Santhanam A., Hinton D., Choi D., Katusic Z. 2013. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 127, 691–700.
Lundberg J., Weitzberg E. 2022. Nitric oxide signaling in health and disease. Cell. 185, 2853–2878.
Tieu K., Ischiropoulos H., Przedborski S. 2003. Nitric oxide and reactive oxygen species in parkinson’s disease. IUBMB Life. 55, 329–335.
He X., Yan N., Chen X., Qi Y., Yan Y., Cai Z. 2016. Hydrogen sulfide down-regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacol. Reports. 68, 975–982.
Tyagi N., Moshal K., Sen U., Vacek T., Kumar M., Hughes W., Kundu S., Tyagi S. 2009. H2S protects against methionine–induced oxidative stress in brain endothelial cells. Antioxid. Redox Signal. 11, 25–33.
Kesherwani V., Nelson K., Agrawal S. 2013. Effect of sodium hydrosulphide after acute compression injury of spinal cord. Brain Res. 1527, 222–229.
Logsdon A., Schindler A., Meabon J., Yagi M., H-erbert M., Banks W., Raskind M. Marshall D., Keene C., Perl D., Peskind E., Cook D. 2020. Nitric oxide synthase mediates cerebellar dysfunction in mice exposed to repetitive blast-induced mild traumatic brain injury. Sci. Rep. 10, 9420.
Rodkin S., Kovaleva V., Berezhnaya E., Neginskaya M., Uzdensky A. 2019. Ca2+- and NF-κB-dependent generation of NO in the photosensitized neurons and satellite glial cells. J. Photochem. Photobiol. B. 199, 111603.
Dobson C. 2003. Protein folding and misfolding. Nature. 426, 884–890.
Morán Luengo T., Mayer M., Rüdiger S. 2019. The Hsp70–Hsp90 chaperone cascade in protein folding. Trends Cell Biol. 29, 164–177.
Naeem A., Fazili N. 2011. Defective protein folding and aggregation as the basis of neurodegenerative diseases the darker aspect of proteins. Cell Biochem. Biophys. 61, 237–250.
Zheng Q., Huang T., Zhang L., Zhou Y., Luo H., Xu H., Wang X. 2016. Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front. Aging Neurosci. 8, 303.
Meng F., Yao D., Shi Y., Kabakoff J., Wu W., Reicher J., Ma Y., Moosmann B., Masliah E., Lipton S., Gu, Z. 2011. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol. Neurodegener. 6, 34.
Chen X., Guan T, Li C., Shang H., Cui L., Li X., Kong J. 2012. SOD1 aggregation in astrocytes following ischemia/reperfusion injury a role of NO-mediated S-nitrosylation of protein disulfide isomerase (PDI). J. Neuroinflammation. 9, 237.
Xu B., Jin C., Deng Y., Liu W., Yang T., Feng S., Xu Z. 2014. Alpha-synuclein oligomerization in manganese-induced nerve cell injury in brain slices a role of NO-mediated s-nitrosylation of protein disulfide isomerase. Mol. Neurobiol. 50, 1098–1110.
Wang H., Shi X., Qiu M., Lv S., Liu H. 2020. Hydrogen Sulfide plays an important protective role through influencing endoplasmic reticulum stress in diseases. Int. J. Biol. Sci. 16, 264–271.
Vandiver M., Paul B., Xu R., Karuppagounder S., Rao F., Snowman A., Seok Ko H., Il Lee Y., Dawson V., Dawson T., Sen N., Snyder S. 2013. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 4, 1626.
Tapias V. 2019. Editorial mitochondrial dysfunction and neurodegeneration. Front. Neurosci. 13, 1372.
Rachek L., Grishko V., LeDoux S., Wilson G. 2006. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic. Biol. Med. 40, 754–762.
Luo Y., Yang X., Zhao S., Wei C., Yin Y., Liu T., Jiang S., Xie J., Wan X., Mao M., Wu J. 2013. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 63, 826–831.
Guo W., Kan J., Cheng Z., Chen J., Shen Y., Xu J., Wu D., Zhu Y. 2012. Hydrogen sulfide as an endogenous modulator in mitochondria and mitochondria dysfunction. Oxid. Med. Cell. Longev. 9, 878052.
Wang J., Medress Z., Barres B. 2012. Axon degeneration molecular mechanisms of a self-destruction pathway. J. Cell Biol. 196, 7–18.
Koeberle P., Ball A. 1999. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp. Neurol. 158, 366–381.
Tang X., Lan M., Zhang M., Yao Z. 2017.Effect of nitric oxide to axonal degeneration in multiple sclerosis via downregulating monocarboxylate transporter 1 in oligodendrocytes. Nitric Oxide. 67, 75–80.
Smith K., Kapoor R., Hall S. 2001. Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol. 49, 470–476.
Liñares D., Taconis M., Maña P., Correcha M., Fordham S., Staykova M., Willenborg D. 2006. neuronal nitric oxide synthase plays a key role in CNS demyelination. J. Neurosci. 26, 12 672–12 681.
Keswani S., Bosch-Marcé M., Reed N., Fischer A, Semenza G., Höke A. 2011. Nitric oxide prevents axonal degeneration by inducing HIF-1–dependent expression of erythropoietin. Proc. Natl. Acad. Sci. USA. 108, 4986–4990.
Cooke R., Mistry R., Challiss R., Straub V. 2013. Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy. J. Neurosci. 33, 5626–5637.
Xu K., Wu F, Xu K., Li Z., Wei X., Lu Q., Jiang T., Wu F., Xu X., Xiao J., Chen D., Zhang H. 2018. NaHS restores mitochondrial function and inhibits autophagy by activating the PI3K/Akt/mTOR signalling pathway to improve functional recovery after traumatic brain injury. Chem. Biol. Interact. 286, 96–105.
Pchitskaya E., Bezprozvanny I. 2020. Dendritic spines shape analysis-classification or clusterization? Perspective. Front. Synaptic Neurosci. 12, 31.
Luo C., Lin Y., Qian X., Tang Y., Zhou H., Jin X., Ni H., Zhang F., Qin C., Li F., Zhang Y., Wu H., Chang L., Zhu D. 2014. Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J. Neurosci. 34, 13535–13548.
Zhang Y., Zhu Z., Liang H., Zhang L., Zhou Q., Ni H., Luo C., Zhu D. 2018. nNOS-CAPON interaction mediates amyloid-β-induced neurotoxicity especially in the early stages. Aging Cell. 17, e12754.
Mir S., Sen T., Sen N. 2014. Cytokine-induced GAPDH sulfhydration affects PSD95 degradation and memory. Mol. Cell. 56, 786–795.
Sen T., Saha P., Jiang T., Sen N. 2020. Sulfhydration of AKT triggers Tau-phosphorylation by activating glycogen synthase kinase 3β in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 117, 4418–4427.
Wang X., Du J., Cui H. 2014. Sulfur dioxide a double-faced molecule in mammals. Life Sci. 98, 63–67.
Rodkin S., Dzreyan V., Bibov M., Ermakov A., Derezina T., Kirichenko E. 2022. NO-dependent mechanisms of p53 expression and cell death in rat’s dorsal root ganglia after sciatic-nerve transection. Biomedicines. 10, 1664.
Nakaya N., Lowe S., Taya Y., Chenchik A., Enikolopov G. 2000. Specific pattern of p53 phosphorylation during nitric oxide-induced cell cycle arrest. Oncogene. 19, 6369–6375.
Wang X., Zalcenstein A., Oren M. 2003. Nitric oxide promotes p53 nuclear retention and sensitizes neuroblastoma cells to apoptosis by ionizing radiation. Cell Death Differ. 10, 468–476.
Sun J., Li X., Gu X., Du H., Zhang G., Wu J., Wang F. 2021. Neuroprotective effect of hydrogen sulfide against glutamate-induced oxidative stress is mediated via the p53/glutaminase 2 pathway after traumatic brain injury. Aging. 13, 7180–7189.
Luo Q., Wu X., Chang W., Zhao P., Nan Y., Zhu X., Katz J., Su D., Liu Z. 2020. ARID1A prevents squamous cell carcinoma initiation and chemoresistance by antagonizing pRb/E2F1/c-Myc-mediated cancer stemness. Cell Death Differ. 27, 1981–1997.
Cui X., Zhang J., Ma P., Myers D., Goldberg I., Sittle-r K., Barb J., Munson P., del Pilar Cintron A., McCoy J., Wang S., Danner R. 2005. cGMP-independent nitric oxide signaling and regulation of the cell cycle. BMC Genomics. 6, 151.
Cai Z., Guo H., Wang C., Wei M., Cheng C., Yang Z., Chen Y., Le W., Li S. 2016. Double-edged roles of nitric oxide signaling on APP processing and amyloid-β production in vitro preliminary evidence from sodium nitroprusside. Neurotox. Res. 29, 21–34.
Kobayashi S., Sasaki T., Katayama T., Hasegawa T., Nagano A., Sato K. 2010. Temporal–spatial expression of presenilin 1 and the production of amyloid-β after acute spinal cord injury in adult rat. Neurochem. Int. 56, 387–393.
Ayton S., Lei P., Hare D.J., Duce J.A., George J.L., Adlard P.A., McLean C., Rogers J.T., Cherny R.A., Finkelstein D.I., Bush A.I. 2015 Parkinson’s disease iron deposition caused by nitric oxide-induced loss of β-amyloid precursor protein. J. Neurosci. 35, 3591–3597.
Tejedo J., Bernabé J., Ramírez R., Sobrino F. 1999. NO induces a cGMP-independent release of cytochrome c from mitochondria which precedes caspase 3 activation in insulin producing RINm5F cells. FEBS Lett. 459, 238–243.
Huang Z., Pinto J., Deng H., Richie J. 2008. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem. Pharmacol. 75, 2234–2244.
Xu C., Zhang M., Zhang G., Yan S., Yan W. 2021. Hydrogen sulfide improves functional recovery in rat traumatic spinal cord injury model by inducing nuclear translocation of NF-E2-related factor 2. Biol. Pharm. Bull. 44, b21-00259.
Ye X., Li Y., Lv B., Qiu B., Zhang S., Peng H., Kong W., Tang C., Huang Y., Du J., Jin H. 2022. Endogenous hydrogen sulfide persulfidates caspase-3 at cysteine 163 to inhibit doxorubicin-induced cardiomyocyte apoptosis. Oxid. Med. Cell. Longev. 20, 6153772.
Wu J., Lipinski M. 2019. Autophagy in neurotrauma good bad or dysregulated. Cells. 8, 693.
Sarkar S., Korolchuk V., Renna M., Imarisio S., Fleming A., Williams A., Garcia-Arencibia M., Rose C., Luo S., Underwood B., Kroemer G., O’Kane C., Rubinsztein D. 2011. Complex inhibitory effects of nitric oxide on autophagy. Mol. Cell. 43, 19–32.
Cervia D., Perrotta C., Moscheni C., De Palma C. 2013. Nitric oxide and sphingolipids control apoptosis and autophagy with a significant impact on Alzheimer’s disease. J. Biol. Regul. Homeost. Agents. 27, 11–22.
Li L., Jiang H., Li Y., Guo Y. 2015. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J. Biomed. Sci. 22, 50.
Zhou J., Jin Y., Lei Y., Liu T., Wan Z., Meng H., Wang H. 2020. Ferroptosis is regulated by mitochondria in neurodegenerative diseases. Neurodegener. Dis. 20, 20–34.
Feng Z., Min L., Chen H., Deng W., Tan M., Liu H., Hou J. 2021. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 43, 101984.
Yu Y., Li X., Wu X., Li X., Wei J., Chen X., Sun Z., Zhang Q. 2023. Sodium hydrosulfide inhibits hemin-induced ferroptosis and lipid peroxidation in BV2 cells via the CBS/H2S system. Cell. Signal. 104, 110594.
Hu X., Chen H., Xu H., Wu Y., Wu C., Jia C., Li Y., Sheng S., Xu C., Xu H., Ni W., Zhou, K. 2020. Role of pyroptosis in traumatic brain and spinal cord injuries. Int. J. Biol. Sci. 16, 2042–2050.
Chen X., Huang X., Liu C., Li S., Yang Z., Zhang F., Chen X., Shan H., Tao L., Zhang M. 2022. Surface-fill H2S-releasing silk fibroin hydrogel for brain repair through the repression of neuronal pyroptosis. Acta Biomater. 154, 259–274.
Godínez-Rubí M., Rojas-Mayorquín A., Ortuño-Sahagún D. 2013. Nitric oxide donors as neuroprotective agents after an ischemic stroke-related inflammatory reaction. Oxid. Med. Cell. Longev. 16, 297357.
Fernandez P., Pozo-Rodrigalvarez A., Serrano J., Martinez-Murillo R. 2010. Nitric oxide target for therapeutic strategies in alzheimers disease. Curr. Pharm. Des. 16, 2837–2850.
Singh S., Das T., Ravindran A. Chaturvedi R.K. Shukla Y. Agarwal A.K. Dikshit M. 2005. Involvement of nitric oxide in neurodegeneration a study on the experimental models of Parkinson’s disease. Redox Rep. 10, 103–109.
Lu D., Mahmood A., Zhang R., Li Y. 2003. Chopp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DETA/NONOate a nitric oxide donor after traumatic brain injury in rats. J. Neurosurg. 99, 351–361.
Khan M., Im Y., Shunmugavel A., Gilg A., Dhindsa R., Singh A., Singh I. 2009. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J. Neuroinflammation. 6, 32.
Pannu R., Singh I. 2006. Pharmacological strategies for the regulation of inducible nitric oxide synthase neurodegenerative versus neuroprotective mechanisms. Neurochem. Int. 49, 170–182.
Lai Y., Tian Y., You X., Du J., Huang J. 2022. Effects of sphingolipid metabolism disorders on endothelial cells. Lipids Health Dis. 21, 101.
Pannu R., Won J., Khan M., Singh A., Singh I. 2004. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-γ-mediated inducible nitric oxide synthase gene expression implications for neuroinflammatory diseases. J. Neurosci. 24, 5942–5954.
Jia J., Xiao Y., Wang W., Qing L., Xu Y., Song H., Zhen X., Ao G., Alkayed N., Cheng J. 2013. Differential mechanisms underlying neuroprotection of hydrogen sulfide donors against oxidative stress. Neurochem. Int. 62, 1072–1078.
Wang R., Wu X., Tian Z., Hu T., Cai C., Wu G., Jian-g G., Liu B. 2023. Sustained release of hydrogen sulfide from anisotropic ferrofluid hydrogel for the repair of spinal cord injury. Bioact. Mater. 23, 118–128.
Liu Y., Pan L., Jiang A., Yin M. 2018. Hydrogen sulfide upregulated lncRNA CasC7 to reduce neuronal cell apoptosis in spinal cord ischemia-reperfusion injury rat. Biomed. Pharmacother. 98, 856–862.
Asimakopoulou A., Panopoulos P., Chasapis C., Coletta C., Zhou Z., Cirino G, Giannis A., Szabo C., Spyroulias G., Papapetropoulos A. 2013. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 169, 922–932.
Wang F., Zhou H., Zhang X. 2022. SAM a cystathionine beta-synthase activator promotes hydrogen sulfide to promote neural repair resulting from massive cerebral infarction induced by middle cerebral artery occlusion. Metab. Brain Dis. 37, 1641–1654.
Funding
The work was supported by the project Nauka-2030.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving human participants or animals performed by any of the authors.
Additional information
Translated by E. Makeeva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodkin, S.V., Nwosu, C.D. Role of Nitric Oxide and Hydrogen Sulfide in Neuronal and Glial Cell Death in Neurodegenerative Processes. Biochem. Moscow Suppl. Ser. A 17, 223–242 (2023). https://doi.org/10.1134/S1990747823050069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747823050069