Abstract—
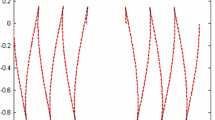
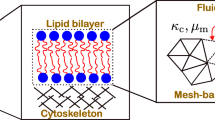
Various membrane inclusions can induce deformations of lipid bilayer membranes. The characteristic length of deformation propagation along the membrane is about several nanometers. Overlapping of deformations induced by different membrane inclusions leads to their effective lateral interaction. The interaction energy can be calculated within the framework of an adequate theory of elasticity. However, in practice, such a calculation can be carried out in an analytical form only for effectively one-dimensional systems, for example, those with translational or rotational symmetry. In the general case of systems with low symmetry, the problem cannot be solved analytically. We theoretically considered the interaction of two cylindrical transmembrane peptides mediated by membrane deformations. The interaction energies were obtained by numerical minimization of the elastic energy functional. In addition, we calculated the interaction energies in a one-dimensional approximation, assuming that the system possesses the translational symmetry. It was shown that the one-dimensional approximation quite well reproduces the results of exact numerical calculations in lipid bilayers of various thicknesses and rigidities.


Similar content being viewed by others
REFERENCES
Kozlovsky Y., Kozlov M.M. 2003. Membrane fission: Model for intermediate structures. Biophys. J. 85, 85–96.
Martens S., Kozlov M.M., McMahon H.T. 2007. How synaptotagmin promotes membrane fusion. Science 316, 1205–1208.
Chernomordik L., Kozlov M.M., Zimmerberg J. 1995. Lipids in biological membrane fusion. J. Membr. Biol. 146, 1–14.
Kozlovsky Y., Kozlov M.M. 2002. Stalk model of membrane fusion: Solution of energy crisis. Biophys. J. 82, 882–895.
Pannuzzo M., McDargh Z.A., Deserno M. 2018. The role of scaffold reshaping and disassembly in dynamin driven membrane fission. Elife. 7, e39441.
Kozlovsky Y., Chernomordik L.V., Kozlov M.M. 2002. Lipid intermediates in membrane fusion: Formation, structure, and decay of hemifusion diaphragm. Biophys. J. 83, 2634–2651.
Helfrich W. 1973. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Naturforsch. C. 28, 693–703.
Hamm M., Kozlov M.M. 2000. Elastic energy of tilt and bending of fluid membranes. Eur. Phys. J. E. 3, 323–335.
Terzi M.M., Deserno M. 2017. Novel tilt-curvature coupling in lipid membranes. J. Chem. Phys. 147, 084702.
Pinigin K.V., Kuzmin P.I., Akimov S.A., Galim-zyanov T.R. 2020. Additional contributions to elastic energy of lipid membranes: Tilt-curvature coupling and curvature gradient. Phys. Rev. E. 102, 042406.
Kondrashov O.V., Galimzyanov T.R., Pavlov K.V., Kotova E.A., Antonenko Y.N., Akimov S.A. 2018. Membrane elastic deformations modulate gramicidin A transbilayer dimerization and lateral clustering. Biophys. J. 115, 478–493.
Kondrashov O.V., Galimzyanov T.R., Jiménez-Munguía I., Batishchev O.V., Akimov S.A. 2019. Membrane-mediated interaction of amphipathic peptides can be described by a one-dimensional approach. Phys. Rev. E. 99, 022401.
Perrin Jr. B.S., Fu R., Cotten M.L., Pastor R.W. 2016. Simulations of membrane-disrupting peptides II: AMP piscidin 1 favors surface defects over pores. Biophys. J. 111, 1258–1266.
Chen C.H., Wiedman G., Khan A., Ulmschneider M.B. 2014. Absorption and folding of melittin onto lipid bilayer membranes via unbiased atomic detail microsecond molecular dynamics simulation. Biochim. Biophys. Acta. 1838, 2243–2249.
Bories F., Constantin D., Galatola P., Fournier J.B. 2018. Coupling between inclusions and membranes at the nanoscale. Phys. Rev. Lett. 120, 128104.
Lin X., Gorfe A.A., Levental I. 2018. Protein partitioning into ordered membrane domains: Insights from simulations. Biophys. J. 114, 1936–1944.
Park S., Yeom M.S., Andersen O.S., Pastor R.W., Im W. 2019. Quantitative characterization of protein–lipid interactions by free energy simulation between binary bilayers. J. Chem. Theor. Comput. 15, 6491–6503.
Molotkovsky R.J., Akimov S.A. 2009. Calculation of the line tension in various models of the lipid bilayer pore edge. Biol. Membrany (Rus.). 26, 149–158.
Karpunin D.V., Akimov S.A., Frolov V.A. 2005. Pore formation in lipid membranes containing lysolipids and cholesterol. Biol. Membrany (Rus.). 22, 429–432.
Israelachvili J. 2011. Intermolecular and Surface Forces. New York: Academic Press, ISBN 9 780 123 919 274.
Nagle J.F., Wilkinson D.A. 1978. Lecithin bilayers. Density measurement and molecular interactions. Biophys. J. 23, 159–175.
Kondrashov O.V., Akimov S.A. 2022. Regulation of antimicrobial peptide activity via tuning deformation fields by membrane-deforming inclusions. Int. J. Mol. Sci. 23, 326.
Rawicz W., Olbrich K.C., McIntosh T., Needham D., Evans E. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79, 328–339.
Hu M., de Jong D.H., Marrink S.J., Deserno M. 2013. Gaussian curvature elasticity determined from global shape transformations and local stress distributions: A comparative study using the MARTINI model. Faraday Discuss. 161, 365–382.
Funding
The work was supported by the Russian Science Foundation (project no. 22-24-00834).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by S. Akimov
Rights and permissions
About this article
Cite this article
Kondrashov, O.V., Akimov, S.A. Lateral Interaction of Cylindrical Transmembrane Peptides in a One-Dimensional Approximation. Biochem. Moscow Suppl. Ser. A 16, 127–134 (2022). https://doi.org/10.1134/S1990747822030060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747822030060