Abstract
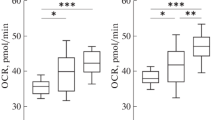
Despite the active study of T-lymphocyte mitochondria under pathology, data on the organelles’ condition in healthy people are not presented in the available literature. The aim of this work was to assess mitochondrial condition in CD4+ and CD8+ T-lymphocytes of healthy subjects. In both cell subsets, the mitochondrial mass and mitochondrial membrane potential, as well as the content of the mitochondrial-activity regulator (PGC-1α), were determined by flow cytometry. Naive and memory cells, as well as resting and cycling lymphocytes were compared. It was shown for the first time that in healthy subjects, mitochondrial condition differs between CD4+ and CD8+ T-lymphocytes. Despite the cell maturation stage or resting/cycling status, the mass and membrane potential of mitochondria in CD4+ T-lymphocytes significantly exceed those in CD8+ T-cells. Both CD4+ and CD8+ T-lymphocytes have higher mitochondrial mass, mitochondrial membrane potential, and PGC-1α content in memory cells and cycling elements. Furthermore in healthy individuals, the relationship between the level of the mitochondrial activity regulator and the indices of mitochondrial condition differ significantly between CD4+ and CD8+ T-lymphocytes: in CD4+ T-cells, the PGC-1α content is directly related to the mass of mitochondria, but in CD8+ T-lymphocytes, it is directly related with the organelles’ transmembrane potential.



Similar content being viewed by others
REFERENCES
Ahrends, T., Spanjaard, A., Pilzecker, B., Babala, N., Bovens, A., Xiao, Y., Jacobs, H. and Borst, J., CD4(+) T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness, Immunity, 2017, vol. 47, p. 848.
Bengsch, B., Johnson, A.L., Kurachi, M., Odorizzi, P.M., Pauken, K.E., Attanasio, J., Stelekati, E., McLane, L.M., Paley, M.A., Delgoffe, G.M., and Wherry, E.J., Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion, Immunity, 2016, vol. 45, p. 358.
Callender, L.A., Carroll, E.C., Bober, E.A., Akbar, A.N., Solito, E., and Henson, S.M., Mitochondrial mass governs the extent of human T cell senescence, Aging Cell, 2020, vol. 19, article ID e13067.
Cheng, C.F., Ku, H.C., and Lin, H., PGC-1alpha as a pivotal factor in lipid and metabolic regulation, Int. J. Mol. Sci., 2018, vol. 19, p. 3447.
Cottet-Rousselle, C., Ronot, X., Leverve, X., and Mayol., J.F., Cytometric assessment of mitochondria using fluorescent probes, Cytometry A, 2011, vol. 79, p. 405.
Farber, D.L., Yudanin, N.A., and Restifo, N.P., Human memory T cells: generation, compartmentalization and homeostasis, Nat. Rev. Immunol., 2014, vol. 14, p. 24.
Fernandez-Marcos, P.J. and Auwerx, J., Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis, Am. J. Clin. Nutr., 2011, vol. 93, p. 884S.
Jones, N., Cronin, J.G., Dolton, G., Panetti, S., Schauenburg, A.J., Galloway, S.A.E., Sewell, A.K., Cole, D.K., Thornton, C.A., and Francis, N.J., Metabolic adaptation of human CD4+ and CD8+ T-cells to T-cell receptor-mediated stimulation, Front. Immunol., 2017, vol. 8, p. 1516.
Kaech, S.M. and Wherry, E.J., Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection, Immunity, 2007, vol. 27, p. 393.
MacIver, N.J., Michalek, R.D., and Rathmell, J.C., Metabolic regulation of T lymphocytes, Annu. Rev. Immunol., 2013, vol. 31, p. 259.
McKinney, E.F. and Smith, K.G.C., Metabolic exhaustion in infection, cancer and autoimmunity, Nat. Immunol., 2018, vol. 19, p. 213.
McLane, L.M., Abdel-Hakeem, M.S., and Wherry, E.J., CD8 T cell exhaustion during chronic viral infection and cancer, Annu. Rev. Immunol., 2019, vol. 37, p. 457.
Mumprecht, S., Schurch, C., Schwaller, J., Solentha-ler, M., and Ochsenbein, A.F., Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression, Blood, 2009, vol. 114, p. 1528.
Nicoli, F., Papagno, L., Frere, J.J., Cabral-Piccin, M.P., Clave, E., Gostick, E., Toubert, A., Price, D.A., Caputo, A., and Appay, V., Naive CD8(+) T-cells engage a versatile metabolic program upon activation in humans and differ energetically from memory CD8(+) T-cells, Front. Immunol., 2018, vol. 9, p. 2736.
Novy, P., Quigley, M., Huang, X., and Yang, Y., CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses, J. Immunol., 2007, vol. 179, p. 8243.
Phan, A.T., Doedens, A.L., Palazon, A., Tyrakis, P.A., Cheung, K.P., Johnson, R.S. and Goldrath, A.W., Constitutive glycolytic metabolism supports CD8(+) T cell effector memory differentiation during viral infection, Immunity, 2016, vol. 45, p. 1024.
Ponnappan, S. and Ponnappan, U., Aging and immune function: molecular mechanisms to interventions, Antioxid. Redox. Signal., 2011, vol. 14, p. 1551.
Presley, A.D., Fuller, K.M., and Arriaga, E.A., MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection, J. Chromatogr. B Anal. Technol. Biomed. Life Sci., 2003, vol. 793, p. 141.
Rogers, P.R., Dubey, C., and Swain, S.L., Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen, J. Immunol., 2000, vol. 164, p. 2338.
Saeidi, A., Zandi, K., Cheok, Y.Y., Saeidi, H., Wong, W.F., Lee, C.Y.Q., Cheong, H.C., Yong, Y.K., Larsson, M., and Shankar, E.M., T-cell exhaustion in chronic infections: Reversing the state of exhaustion and reinvigorating optimal protective immune responses, Front. Immunol., 2018, vol. 9, p. 2569.
Sallusto, F., Lenig, D., Forster, R., Lipp, M., and Lanzavecchia, A., Two subsets of memory T lymphocytes with distinct homing potentials and effector functions, Nature, 1999, vol. 401, p. 708.
Spinelli, J.B. and Haigis, M.C., The multifaceted contributions of mitochondria to cellular metabolism, Nat. Cell Bi-l., 2018, vol. 20, p. 745.
Sukumar, M., Liu, J., Mehta, G.U., Patel, S.J., Roychoudhuri, R., Crompton, J.G., Klebanoff, C.A., Ji, Y., Li, P., Yu, Z., Whitehill, G.D., Clever, D., Eil, R.L., Palmer, D.C., Mitra, S., Rao, M., et al., Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy, Cell Metab., 2016, vol. 23, p. 63.
Sun, H. and Li, X., Metabolic reprogramming in resting and activated immune cells, Metabolomics (Los Angel), 2017, vol. 7, p. 188.
Toma, G., Lemnian, I.M., Karapetian, E., Grosse, I., and Seliger, B., Transcriptional analysis of total CD8(+) T cells and CD8(+)CD45RA(-) memory T cells from young and old healthy blood donors, Front. Immunol., 2022, vol. 13, p. 806906. https://doi.org/10.3389/fimmu.2022.806906
Van der Windt, G.J., Everts, B., Chang, C.H., Curtis, J.D., Freitas, T.C., Amiel, E., Pearce, E.J., and Pearce, E.L., Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development, Immunity, 2012, vol. 36, p. 68.
Van der Windt, G.J., O’Sullivan, D., Everts, B., Huang, S.C., Buck, M.D., Curtis, J.D., Chang, C.H., Smith, A.M., Ai, T., Faubert, B., Jones, R.G., Pearce, E.J., and Pearce, E.L., CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, p. 14336.
Yang, P., Ma, J., Yang, X., and Li, W., Peripheral CD4+ naive/memory ratio is an independent predictor of survival in non-small cell lung cancer, Oncotarget, 2017, vol. 8, p. 83650.
Younes, S.A., Talla, A., Pereira Ribeiro, S., Saidakova, E.V., Korolevskaya, L. B., Shmagel, K. V., Shive, C. L., Freeman, M. L., Panigrahi, S., Zweig, S., Balderas, R., Margolis, L., Douek, D. C., Anthony, D. D., Pandiyan, P., et al., Cycling CD4+ T cells in HIV-infected immune nonresponders have mitochondrial dysfunction, J. Clin. Invest., 2018, vol. 128, p. 5083.
Yu, F., Hao, Y., Zhao, H., Xiao, J., Han, N., Zhang, Y., Dai, G., Chong, X., Zeng, H. and Zhang, F., Distinct mitochondrial disturbance in CD4+T and CD8+T cells from HIV-infected patients, J. Acquired Immune Defic. Syndr., 2017, vol. 74, p. 206.
Yu, Y.R., Imrichova, H., Wang, H., Chao, T., Xiao, Z., Gao, M., Rincon-Restrepo, M., Franco, F., Genolet, R., Cheng, W.C., Jandus, C., Coukos, G., Jiang, Y.F., Locasale, J.W., Zippelius, A., Liu, P.S., et al., Disturbed mitochondrial dynamics in CD8(+) TILs reinforce T cell exhaustion, Nat. Immunol., 2020, vol. 21, p. 1540.
Zhu, J., Yamane, H., and Paul, W.E., Differentiation of effector CD4 T cell populations (*), Annu. Rev. Immunol., 2010, vol. 28, p. 445.
Funding
The work was performed as part of the state order “The Role of CD4+ Memory T-cell Metabolism in Disrupted Immunity Regeneration in HIV-Infected Patients Undergoing Antiretroviral Therapy,” state reg. no. 121112500044-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of compliance with standards of research involving humans as subjects. All studies involving people were carried out in accordance with the principles of institutional and/or national committees on biomedical ethics and the Declaration of Helsinki of 1964 and its subsequent emendations or comparable ethical principles. Each participant provided voluntary informed consent. The work was approved by the Local Bioethics Committee of the Perm Regional Center for Prevention and Control of AIDS and Infectious Diseases, reg. no. IRB00008964.
Additional information
Translated by A. Deryabina
Rights and permissions
About this article
Cite this article
Korolevskaya, L.B., Saidakova, E.V., Shmagel, N.G. et al. Assessment of the Mitochondrial Condition in CD4+ and CD8+ T-Lymphocytes from Healthy Subjects. Cell Tiss. Biol. 16, 470–477 (2022). https://doi.org/10.1134/S1990519X22050054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X22050054