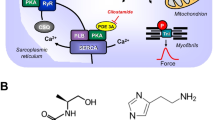
Abstract
Hydrogen sulfide (H2S) is an endogenously synthesized gaseous transmitter that participates in the regulation of the cardiovascular system and has a cardioprotective effect under ischemia–reperfusion conditions. Here, we studied possible mechanisms of the interaction of H2S and muscarinic acetylcholine receptors in the regulation of mice atrium contractility in the isometric conditions. We show that sodium hydrosulfide (NaHS), an exogenous donor of H2S, caused dose-dependent and reversible depression of the contractile force in the concentration range from 1 μM to 5 mM. The negative inotropic effect of NaHS did not change after the activation of muscarinic acetylcholine receptors by carbachol. However, we observed that the negative inotropic effect of carbachol increased after preliminary application of NaHS. The application of the reducing agent dithiothreitol did not change the effects of carbachol, which indicated that the effects of NaHS was not related to a direct action on the disulfide bonds of the receptor’s protein subunits. The increased effects of carbachol after NaHS application were not prevented by the inhibition of intracellular signaling pathway that mediated activation of M-cholinergic receptors, including adenylate cyclase, guanylate cyclase, and NO-synthase. However, an increase in the carbachol negative inotropic effect was not observed when ATP-dependent potassium channels were inhibited by glibenclamide. In its turn, activation of ATPdependent potassium channels by diazoxide resulted in an increase in carbachol negative inotropic action in the atrial myocardium of mice similar to the effect of NaHS. Our data indicate that the enhanced negative inotropic effect of carbachol under the action of H2S in the mouse atrium was mediated by the activation of ATP-dependent potassium channels.
Similar content being viewed by others
Abbreviations
- H2S:
-
hydrogen sulfide
- NaHS:
-
sodium hydrosulfide
- CO:
-
carbon monoxide
- NO:
-
nitric oxide
- CBS:
-
cystathionine β-synthase
- CGL:
-
cystathionine γ-lyase
- 3MST:
-
3-mercaptopyruvate sulfurtransferase
- K(ATP)-channels:
-
ATPdependent potassium channels
- M-AChR:
-
muscarinic acetylcholine receptors
- M2-AChR and M3-AChR:
-
muscarinic acetylcholine receptors of type 2 and type 3, respectively
- cGMP:
-
cyclic guanosine monophosphate
References
Sitdikova, G.F. and Zefirov, A.L., Priroda (Moscow), 2010, no. 9, pp. 29–37.
Wang, R., Physiol. Rev., 2012, no. 92, pp. 791–896.
Gerasimova, E., Lebedeva, J., Yakovlev, A., Zefirov, A., Giniatullin, R., and Sitdikova, G., J. Neurosci., 2015, vol. 303, pp. 577–585.
Shafigullin, M.Y., Zefirov, R.A., Sabirullina, G.I., Zefirov, A.L., and Sitdikova, G.F., Bull. Exp. Biol. Med., 2014, vol. 157, no. 3, pp. 302–306.
Wang, M.J., Cai, W.J., and Zhu, Y.C., Life Sci., 2016, vol. 153, pp. 188–197.
Polhemus, D.J. and Lefer, D.J., Circ. Res., 2014, vol. 114, pp. 730–737.
Elsey, D.J., Fowkes, R.C., and Baxter, G.F., Cell Biochem. Funct., 2010, vol. 28, no. 2, pp. 95–106.
Yong, Q.C., Pan, T.T., Hu, L.F., and Bian, J.S., J. Mol. Cell. Cardiol., 2008, vol. 44, no. 4, pp. 701–710.
Sun, Y.G., Cao, Y.X., Wang, W.W., Ma, S.F., Yao, T., and Zhu, Y.C., Cardiovasc. Res., 2008, vol. 79, pp. 632–641.
Xu, M., Wu, Y.M., Li, Q., Wang, F.W., and He, R.R., Sheng Li Xue Bao, 2007, vol. 59, no. 2, pp. 215–220.
Sitdikova, G.F., Khaertdinov, N.N., and Zefirov, A.L., Bull. Exp. Biol. Med., 2011, vol. 151, no. 2, pp. 163–166.
Khaertdinov, N.N., Ahmetshina, D.R., Zefirov, A.L., and Sitdikova, G.F., Biochemistry (Moscow) Suppl. Ser. A: Membrane Cell Biol., 2013, vol. 7, pp. 52–57.
Khaertdinov, N.N., Lifanova, A.S., Gizzatullin, A.R., and Sitdikova, G.F., Genes and Cells, 2015, vol. 10, pp. 103–105.
Lifanova, A.S., Khaertdinov, N.N., Zakharov, A.V., Gizzatullin, A.R., and Sitdikova, G.F., Genes and Cells, 2014, vol. 9, no. 3, pp. 94–98.
Lifanova, A., Khaertdinov, N., and Sitdikova, G., BioNanoScience, 2017, vol. 7, no. 2, pp. 306–308.
Coletta, C., Papapetropoulos, A., Erdelyi, K., Olah, G., Módis, K., Panopoulos, P., Asimakopoulou, A., Gerö, D., Sharina, I., Martin, E., and Szabo, C., Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 23, pp. 9161–9166.
Aydinoglu, F., Dalkir, F.T., Demirbag, H.O., and Ogulener, N., Nitric Oxide, 2017, vol. 70, pp. 51–58.
Sitdikova, G.F., Fuchs, R., Kainz, V., Weiger, T.M., and Hermann, A., Front. Physiol., 2014, vol. 5, no. 431, pp. 1–15.
DeLeon, E.R., Stoy, G.F., and Olson, K.R., Anal. Biochem., 2012, vol. 421, no. 1, pp. 203–207.
Geng, B., Yang, J., Qi, Y., Zhao, J., Pang, Y., Du, J., and Tang, C., Biochem. Biophys. Res. Commun., 2004, vol. 313, pp. 362–368.
Abramochkin, D.V., Moiseenko, L.S., and Kuzmin, V.S., Bull. Exp. Biol. Med., 2009, vol. 147, no. 6, pp. 683–686.
Hara, Y., Ike, A., Tanida, R., Okada, M., and Yamawaki, H., J. Pharmacol. Exp. Ther., 2009, vol. 331, no. 3, pp. 808–815.
Harvey, R.D. and Belevych, A.E., Br. J. Pharmacol., 2003, vol. 139, no. 6, pp. 1074–1084.
Balligand, J.L., Kelly, R.A., Marsden, P.A., Smith, T.W., and Michel, T., Proc. Natl. Acad. Sci. U.S.A., 1993, vol. 90, pp. 347–351.
Wang, Z., Shi, H., and Wang, H., Br. J. Pharmacol., 2004, vol. 142, no. 3, pp. 395–408.
Moncada, S., Palmer, R.M.J., and Higgs, E.A., Pharmacol. Rev., 1991, vol. 43, pp. 109–142.
Lu, J., Zang, W.J., Yu, X.J., Jia, B., Chorvatova, A., and Sun, L., Eur. J. Pharmacol., 2006, vol. 549, pp. 133–139.
Ribalet, B., John, S.A., Xie, L.H., and Weiss, J.N., J. Mol. Cell. Cardiol., 2005, vol. 39, pp. 71–77.
Jiang, B., Tang, G., Cao, K., Wu, L., and Wang, R., Antioxid. Redox Signal., 2010, vol. 12, no. 10, pp. 1167–1178.
Mustafa, A.K., Gadalla, M.M., and Snyder, S.H., Sci. Signal., 2009, vol. 2, no. 68, pp. 1–17.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Blokhina, N.N. Khaertdinov, A.L. Zefirov, G.F. Sitdikova, 2018, published in Neirokhimiya, 2018, Vol. 35, No. 4, pp. 294–300.
Rights and permissions
About this article
Cite this article
Blokhina, A.S., Khaertdinov, N.N., Zefirov, A.L. et al. Interaction between Hydrogen Sulfide and Muscarinic Receptors in the Regulation of Contractility of the Mouse Atrium. Neurochem. J. 12, 299–304 (2018). https://doi.org/10.1134/S1819712418040025
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712418040025