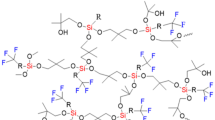
Abstract
Crosslinked polysiloxanes with boron bis(dibenzoylmethanate) complexes used as crosslink junctions of polymer networks are first synthesized, and their physicochemical, mechanical, thermal, and fluorescent properties are studied. It is shown that the polymers under study feature the elastic behavior, possess high thermal and thermo-oxidative stability, and exhibit intense fluorescence in a wide wavelength range (400‒700 nm).








Similar content being viewed by others
REFERENCES
S. Wei, Z. Li, W. Lu, H. Liu, J. Zhang, T. Chen, and B. Z. Tang, Angew. Chemie Int. Ed. 60, 8608 (2021).
G. Ahumada and M. Borkowska, Polymers (Basel) 14, 1118 (2022).
S. Cichosz, A. Masek, and M. Zaborski, Polym. Test. 67, 342 (2018).
C. Calvino, A. Guha, C. Weder, and S. Schrett, Adv. Mater. 30, 1704603 (2018).
X. Du, C. Wang, G. Wu, and S. Chen, Angew. Chemie Int. Ed. 60, 8585 (2021).
L. Birchall, A. Foerster, G. A. Rance, A. Terry, R. D. Wildman, and C. J. Tuck, Sens. Actuators, A 347, 113977 (2022).
J. Alday, A. Mazzeo, and S. Suarez, Inorg. Chim. Acta 510, 119696 (2020).
P.-Z. Chen, L.-Y. Niu, Y.-Z. Chen, and Q.-Z. Yang, Coord. Chem. Rev. 350, 196 (2017).
K. Tanaka and Y. Chujo, NPG Asia Mater. 7, e223 (2015).
A. Loudet and K. Burgess, Chem. Rev. 107, 4891 (2007).
D. Frath, J. Massue, G. Ulrich, and R. Ziessel, Angew. Chemie Int. Ed. 53, 2290 (2014).
D. Li, H. Zhang, and Y. Wang, Chem. Soc. Rev. 42, 8416 (2013).
R. Yoshii, K. Suenaga, K. Tanaka, and Y. Chujo, Chem.-Eur. J. 21, 7231 (2015).
T. Sagawa, F. Ito, A. Sakai, Y. Ogata, K. Tanaka, and H. Ikeda, Photochem. Photobiol. Sci. 15, 420 (2016).
Y. N. Kononevich, M. N. Temnikov, A. A. Korlyukov, A. D. Volodin, P. V. Dorovatovskii, V. A. Sazhnikov, A. A. Safonov, D. S. Ionov, A. A. Ivanov, N. M. Surin, E. A. Svidchenko, and A. M. Muzafarov, ChemPlusChem 85, 1111 (2020).
W. A. Morris, M. Kolpaczynska, and C. L. Fraser, J. Phys. Chem. 120, 22539 (2016).
M. Louis, C. Piñero García, A. Brosseau, C. Allain, and R. Metivier, J. Phys. Chem. Lett. 10, 4758 (2019).
L. Zhang, L.-L. Ma, X. Wang, and X.-Y. Zhao, J. Lumin. 214, 116560 (2019).
M. Louis, R. Sethy, J. Kumar, S. Katao, R. Guillot, T. Nakashima, C. Allain, T. Kawai, and R. Metivier, Chem. Sci. 10, 843 (2019).
C.-T. Poon, W. H. Lam, H.-L. Wong, and V. W.-W. Yam, J. Am. Chem. Soc. 132, 13992 (2010).
Z. Li, D. Wang, D. Ramella, H. Gao, H. Cao, Y. Zhao, Z. Miao, Z. Yang, and W. He, Polym. Chem. 11, 3046 (2020).
M. Zhuang, S. Joshi, H. Sun, T. Batabyal, C. L. Fraser, and J. Kapur, Sci. Rep. 11, 1076 (2021).
Z. Li, Y. Pei, S. Hou, Y. Dai, D. Liu, J. Zhu, Y.-P. Zhu, and X. Liu, Dyes Pigm. 179, 108419 (2020).
Y. N. Kononevich, V. A. Sazhnikov, A. S. Belova, A. A. Korlyukov, A. D. Volodin, A. A. Safonov, G. A. Yurasik, D. S. Ionov, and A. M. Muzafarov, New J. Chem. 43, 13725 (2019).
D. Ionov, G. Yurasik, Y. Kononevich, V. Sazhnikov, A. Muzafarov, and M. Alfimov, Procedia Eng. 168, 341 (2016).
D. S. Ionov, V. A. Sazhnikov, G. A. Yurasik, A. V. Antonov, Y. N. Kononevich, and M. V. Alfimov, High Energy Chem. 49, 183 (2015).
N. M. D. Brown and P. Bladon, J. Chem. Soc. A, No. 526, 526 (1969).
G. A. Reynolds and C. H. Chen, J. Heterocycl. Chem. 22, 657 (1985).
D. Martin, Chem. Rev. 34, 461 (1944).
W. Gerrard and M. F. Lappert, Chem. Rev. 58, 1081 (1958).
A. Barabas, E. Isfan, M. Roman, M. Paraschiv, E. Romas, and A. T. Balaban, Tetrahedron 24, 1133 (1968).
Z. Sui, R. Salto, J. Li, C. Craik, and P. R. Ortiz de Montellano, Bioorg. Med. Chem. 1, 415 (1993).
W.-Y. Shao, Y.-N. Cao, Z.-W. Yu, W.-J. Pan, X. Qiu, X.-Z. Bu, L.-K. An, Z.-S. Huang, L.-Q. Gu, and A. S. C. Chan, Tetrahedron Lett. 47, 4085 (2006).
L. Shi, L. Gao, S. Cai, Q. Xiong, and Z. Ma, Eur. J. Med. Chem. 221, 113528 (2021).
P. Banet, L. Legagneux, P. Hesemann, J. Moreau, L. Nicole, A. Quach, C. Sanchez, and T. Tranthi, Sens. Actuators, B 130, 1 (2008).
X. Chen, X. Zhang, and G. Zhang, Chem. Commun. 51, 161 (2015).
X. Zhang and G. Zhang, Anal. Methods 4, 2641 (2012).
Y. Liang, L. Xu, F. Qu, K. Tang, H. Wang, and W. W. Yu, Polym. Chem. 10, 4818 (2019).
I. Toulokhonova, B. Bjerke-Kroll, and R. West, J. Organomet. Chem. 686, 101 (2003).
A. C. Benniston, G. Copley, A. Harriman, and R. Ryan, J. Mater. Chem. 21, 2601 (2011).
W. Kasprzyk, P. Krzywda, S. Bednarz, and D. Bogdal, RSC Adv. 5, 90473 (2015).
J. Chen, L. Song, Y. Wu, B. Zhao, and J. Deng, ACS Appl. Polym. Mater. 4, 4264 (2022).
A. A. Pakhomov, Yu. N. Kononevich, M. V. Stukalova, E. A. Svidchenko, N. M. Surin, G. V. Cherkaev, O. I. Shchegolikhina, V. I. Martynov, and A. M. Muzafarov, Tetrahedron Lett. 57, 979 (2016).
A. A. Pakhomov, V. B. Mironiuk, Yu. N. Kononevich, A. A. Korlyukov, A. D. Volodin, T. A. Pryakhina, V. I. Martynov, and A. M. Muzafarov, Mendeleev Commun. 27, 363 (2017).
A. A. Pakhomov, E. E. Kim, Yu. N. Kononevich, D. S. Ionov, M. A. Maksimova, V. B. Khalchenia, E. G. Maksimov, A. A. Anisimov, O. I. Shchegolikhina, V. I. Martynov, and A. M. Muzafarov, Dyes Pigm. 203, 110371 (2022).
A. S. Belova, A. G. Khchoyan, T. M. Il’ina, Yu. N. Kononevich, D. S. Ionov, V. A. Sazhnikov, D. A. Khanin, G. G. Nikiforova, V. G. Vasil’ev, and A. M. Muzafarov, Polymers (Basel) 14, 5075 (2022).
E. E. Kim, Yu. N. Kononevich, Y. S. Dyuzhikova, D. S. Ionov, D. A. Khanin, G. G. Nikiforova, O. I. Shchegolikhina, V. G. Vasil’ev, and A. M. Muzafarov, Polymers (Basel) 14, 2554 (2022).
E. E. Kim, Yu. N. Kononevich, A. A. Anisimov, M. I. Buzin, V. G. Vasil’ev, A. A. Korlyukov, D. S. Ionov, D. A. Khanin, E. V. Shtykova, V. V. Volkov, and A. M. Muzafarov, React. Funct. Polym. 164, 104896 (2021).
D. F. Eaton, Pure Appl. Chem. 62, 1631 (1990).
X. Sun, X. Wang, X. Li, J. Ge, Q. Zhang, J. Jiang, and G. Zhang, Macromol. Rapid Commun. 36, 298 (2015).
E. V. Fedorenko, M. K. Behra, and N Kanwishera, J. Fluoresc. 26, 1839 (2016).
A. A. Khrebtov, E. V. Fedorenko, and A. G. Mirochnik, Polymer (Guildf) 256, 125255 (2022).
Funding
This work was supported by the Russian Science Foundation (project no. 18-73-10152). Characterization of compounds by NMR spectroscopy, TGA, and DSC performed using equipment of the Center for Molecule Composition Studies at the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences was supported by the Ministry of Science and Higher Education of the Russian Federation (contract no. 075-03-2023-642). Luminescence lifetimes were measured using equipment of the Center for Collective Use “Structural Diagnostics of Materials” within the framework of State Assignment for the Federal National Research Center “Crystallography and Photonics,” Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Soboleva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, E.E., Il’ina, T.M., Kononevich, Y.N. et al. New Fluorescent Materials Based on Polysiloxanes and Boron Bis(β-diketonates). Polym. Sci. Ser. C 65, 267–276 (2023). https://doi.org/10.1134/S1811238223700376
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1811238223700376