Abstract
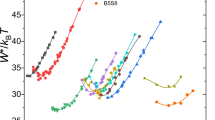
The crystal nucleation in the glass of composition (mol %) 26Li2O · 74SiO2 has been investigated in the cases of homogeneous and heterogeneous nucleation. Parameters of homogeneous nucleation, such as the stationary nucleation rate I st, the time of nonstationary nucleation τ, and the crystal growth rate U, have been determined. The temperature dependences of these parameters have been constructed. The surface energy σ at the nucleus-glass melt interface has been determined, and its temperature dependence has been obtained. The surface energy σ has been evaluated using the graphical method for solving the transcendental equation derived by transforming the relationships for the stationary crystal nucleation rate and the time of nonstationary crystal nucleation. The critical nucleus sizes r* and the free energy of formation of the critical nucleus Φ* have been determined. The heterogeneous nucleation on bubbles specially produced in the glass has been studied. It has been demonstrated that the presence of bubbles in the initial glass does not affect the crystal growth rate and substantially changes the nonstationary nucleation rate. The largest contribution to the change in the nucleation rate is made by “active” bubbles (filled by water vapor) formed in the glasses synthesized with the use of hydrated silicon dioxide.
Similar content being viewed by others
References
Glastechnische Fabrikationsfehler, Jebsen-Marwedel, H. and Brückner, R., Eds., Berlin: Springer, 1980. Translated under the title Vidy braka v proizvodstve stekla, Moscow: Stroiizdat, 1986 [in Russian].
Fiziko-khimicheskie osnovy proizvodstva opticheskogo stekla (Physicochemical Principles of the Manufacture of Optical Glass), Demkina, L.I., Ed., Leningrad: Khimiya, 1976 [in Russian].
Kopsel, D., Boob, M., Opyd, M., and Aigner, M.-L., Nitrogen and Argon in Borosilicate Glass: Solubility, Diffusivity, Residual Gas Concentration, and Behavior of Bubbles in Glass Melts, Containing Both Gases, in Glass-The Challenge for the 21st Century (Proceedings of the 9th ESG Conference, Tren in, Slovakia, 2008), Liska, M., Galusec, D., Klement, R., and Petruskova, V., Eds. (Adv. Mater. Res. (Zuerich, Switz.), 2008, vols. 39–40, pp. 475–480).
Tonarova, V. and Nemec, L., The Bubble Behaviour under Effect of Centrifugal Force, in Glass—The Challenge for the 21st Century (Proceedings of the 9th ESG Conference, Tren in, Slovakia, 2008), Liska, M., Galusec, D., Klement, R., and Petruskova, V., Eds. (Adv. Mater. Res. (Zuerich, Switz.), 2008, vols. 39–40, pp. 475–480).
Nemec, L., Jebava, M., and Cincibusova, P., The Removal of Bubbles from Glass Melts in Horizontal or Vertical Channels with Different Glass Flow Patterns, Ceram.-Silik., 2006, vol. 50, no. 3, pp. 140–152.
Cincibusova, P., Nemec, L., and Brada, J., The Influence of Glass Melt Flow Character on the Bubble Removal Process, in Glass-The Challenge for the 21st Century (Proceedings of the 9th ESG Conference, Tren in, Slovakia, 2008), Liska, M., Galusec, D., Klement, R., and Petruskova, V., Eds. (Adv. Mater. Res. (Zuerich, Switz.), 2008, vols. 39–40, pp. 419–424).
GOST (State Standard) 3522-81: Optical Materials. Method for Determining the Presence of Bubbles, 1982.
Tamman, G., Über die Abhängigkeit der Zahl der Kerne welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur, Z. Phys. Chem., 1998, vol. 25, no. 3, pp. 441–479.
Tabata, K. On the Causer of the Surface Devitrification of Glasses, J. Am. Ceram. Soc., 1927, vol. 10, no. 1, pp. 6–22.
Morey, G.W., The Devitrification of Soda-Lime-Silica Glasses, J. Am. Ceram. Soc., 1930, vol. 13, no. 10, pp. 683–713.
Scott, W.D. and Pask, J.A., Nucleation and Growth of Sodium Disilicate Crystals in Sodium Disilicate Glass, J. Am. Ceram. Soc., 1961, vol. 44, no. 4, pp. 181–187.
Klinsberg, C., Bubble-Induced Nucleation and Crystal Growth in Glass, J. Am. Ceram. Soc., 1964, vol. 47, no. 2, pp. 97–100.
Neely, J.E. and Ernsberger, F.M., Absence of Devitrification of Bubble Surface in Soda-Lime Glasses, J. Am. Ceram. Soc., 1966, vol. 49, no. 7, pp. 396–397.
Bergeron, C.G. and Deluca, J.P., Crystal Growth at Bubble Surface in Glass, J. Am. Ceram. Soc., 1967, vol. 50, no. 2, pp. 116–117.
Mattox, D.M., Contamination—Nucleated Crystal Growth at Bubble Surface in Glass, J. Am. Ceram. Soc., 1967, vol. 50, no. 12, pp. 683–689.
Leko, V.K. and Komarova, L.A., Crystallization of Silica Glasses in Different Gaseous Media, Izv. Akad. Nauk SSSR, Neorg. Mater., 1975, vol. 2, no. 6, pp. 1115–1120.
Leko, V.K., Effects of Physicochemical Processes during Synthesis and Heat Treatment on the Properties of Vitreous Silica, Fiz. Khim. Stekla, 1982, vol. 8, no. 2, pp. 129–148.
Volksch, G. and Heide, K., Dissolved Gases and Minor Component Effects on Glass Crystallization, J. Non-Cryst. Solids, 1997, vol. 219, pp. 119–127.
Sycheva, G.A., Nucleation of Sodium-Zinc Phosphate Crystals in Sodium-Zinc Phosphate Glass, Fiz. Khim. Stekla, 1998, vol. 24, no. 5, pp. 575–581 [Glass Phys. Chem. (Engl. transl.), 1998, vol. 24, no. 5, pp. 406–410].
Davis, M.J. and Ihinger, P., Nucleation and Crystal Growth in Lithium Disilicate Glass, Am. Mineral., 1998, vol. 83, pp. 1008–1015.
Sycheva, G.A., Formation of the Bubble Structure in the 26Li2O · 74SiO2 Glass, Fiz. Khim. Stekla, 2009, vol. 35, no. 3, pp. 342–351 [Glass Phys. Chem. (Engl. transl.), 2009, vol. 35, no. 3, pp. 267–273].
Mel’nichenko, T.D., Rizak, V.M, Mel’nichenko, T.K., and Fedelesh, V.I., Parameters of the Fluctuation Free Volume Theory for Glasses in the Ge-As-Se System, Fiz. Khim. Stekla, 2004, vol. 30, no. 5, pp. 553–564 [Glass Phys. Chem. (Engl. transl.), 2004, vol. 30, no. 5, pp. 406–414].
Filipovich, V.K. and Kalinina, A.M., On the Relation of the Temperature of the Crystal Nucleation Rate in Glasses to the Glass Transition Temperature, Izv. Akad. Nauk SSSR, Neorg. Mater, 1971, vol. 7, no. 10, pp. 1844–1848.
Mel’nichenko, T.D., Rizak, V.M., Mel’nichenko, T.K., and Fedelesh, V.I., Parameters of the Fluctuation Free Volume Theory for Glasses in the Ge-As-Se System, Fiz. Khim. Stekla, 2004, vol. 30, no. 5, pp. 553–564 [Glass Phys. Chem. (Engl. transl.), 2004, vol. 30, no. 5, pp. 406–414].
Gutzov, I. and Toshev, S., The Kinetics of Nucleation and the Formation of Glass-Ceramic Materials, in Advances in Nucleation and Crystallization in Glasses, Hench, L.L. and Freiman, S.W., Eds., Columbus, Ohio, United States: American Ceramic Society, 1971.
Kashchiev, D., Solution of Non-Steady Problem in Nucleation Kinetics, Surf. Sci., 1969, vol. 14, pp. 209–220.
Kalinina, A.M., Fokin, V.M., and Filipovich, V.N., On the Technique for Determining the Parameters Characterizing Crystal Nucleation in Glasses, Fiz. Khim. Stekla, 1976, vol. 2, no. 4, pp. 298–304.
Saltykov, S.N., Stereometricheskaya metallografiya (Stereometric Metallography), Moscow: Metallurgiya, 1970 [in Russian].
Kalinina, A.M., Fokin, V.M., and Filipovich, V.N., Induction Period of Crystal Nucleation in the Li2O · 2SiO2 Glass and Its Temperature Dependence, Fiz. Khim. Stekla, 1977, vol. 3, no. 2, pp. 123–129.
Takahashi, K. and Yoshio, T., Thermodynamic Quantities of Alkali Silicates in the Temperature Range from 25°C to Melting Point, J. Ceram. Soc. Jpn., 1973, vol. 81, no. 940, pp. 524–533.
Sycheva, G.A., Evaluation of the Surface Energy at the Crystal-Glass Interface in Sodium Silicate Glass 46Na2O · 54SiO2, Fiz. Khim. Stekla, 1998, vol. 24, no. 1, pp. 72–81 [Glass Phys. Chem. (Engl. transl.), 1998, vol. 24, no. 1, pp. 47–53].
Sycheva, G.A., Surface Energy at the Crystal Nucleus-Glass Interface in Alkali Silicate Glasses, Fiz. Khim. Stekla, 1998, vol. 24, no. 4, pp. 491–498 [Glass Phys. Chem. (Engl. transl.), 1998, vol. 24, no. 4, pp. 342–347].
Sycheva, G.A., Formation of Bubble Structure and Nucleation in Glass 26Li2O · 74SiO2, in Nanoparticles, Nanostructures, and Nanocomposites (Proceedings of the Topical Meeting of the European Ceramic Society, St. Petersburg, 2006), St. Petersburg, 2006, pp. 27–29.
Filipovich, V.K, Kalinina, A.M., and Sycheva, G.A., Temperature Dependence of the Rate of Nucleation of Needle Crystals in Sodium Silicate Glass, Izv. Akad. Nauk SSSR, Neorg. Mater., 1975, vol. 11, no. 7, pp. 1305–1308.
Kalinina, A.M., Filipovich, V.K., and Sycheva, G.A., Catalyzed Crystallization of Pyroxene Glasses, Fiz. Khim. Stekla, 1981, vol. 7, no. 1, pp. 55–60.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.A. Sycheva, 2009, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Sycheva, G.A. Influence of the presence of bubbles on the parameters of crystal nucleation in the 26Li2O · 74SiO2 glass. Glass Phys Chem 35, 602–612 (2009). https://doi.org/10.1134/S1087659609060091
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659609060091