Abstract
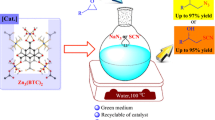
Wet zinc oxide nanopowder was found to be a highly effective catalyst for the cyclization of o-aminobenzamide with aldehydes, followed by dehydrogenation under air atmosphere. The presence of moisture in the catalyst played a crucial role in the selective formation of quinazolin-4(3H)-ones under solvent-free conditions. The procedure is operationally simple, and it does not need toxic or volatile organic solvents and provides excellent yield of the products. Moreover, the catalyst was recycled and reused in three successive reactions without significant loss of activity.


Similar content being viewed by others
REFERENCES
Horton, D.A., Bourne, G.T., and Smythe, M.L., Chem. Rev., 2003, vol. 103, p. 893. https://doi.org/10.1021/cr020033s
Kshirsagar, U.A., Org. Biomol. Chem., 2015, vol. 13, p. 9336. https://doi.org/10.1039/C5OB01379H
Mhaske, S.B. and Argade, N.P., Tetrahedron, 2006, vol. 62, p. 9787. https://doi.org/10.1016/j.tet.2006.07.098
Cagir, A., Jones, S.H., Gao, R., Eisenhauer, B.M., and Hecht, S.M., J. Am. Chem. Soc., 2003, vol. 125, p. 13628. https://doi.org/10.1021/ja0368857
Jiang, J.B., Hesson, D.P., Dusak, B.A., Dexter, D.L., Kang, G.J., and Hamel, E., J. Med. Chem., 1990, vol. 33, p. 1721. https://doi.org/10.1021/jm00168a029
Ozaki, K., Yamada, Y., Oine, T., Ishizuka, T., and Iwasawa, Y., J. Med. Chem., 1985, vol. 28, p. 568. https://doi.org/10.1021/jm50001a006
Grover, G. and Kini, S.G., Eur. J. Med. Chem., 2006, vol. 41, p. 256. https://doi.org/10.1016/j.ejmech.2005.09.002
Abou-Seri, S.M., Abouzid, K., and Abou El Ella, D.A., Eur. J. Med. Chem., 2011, vol. 46, p. 647. https://doi.org/10.1016/j.ejmech.2010.11.045
Wolfe, J.F., Rathman, T.L., Sleevi, M.C., Campbell, J.A., and Greenwood, T.D., J. Med. Chem., 1990, vol. 33, p. 161. https://doi.org/10.1021/jm00163a027
Tang, L., Zhao, X., Zou, G., Zhou, Y., and Yang, X., Asian J. Org. Chem., 2016, vol. 5, p. 335. https://doi.org/10.1002/ajoc.201500512
Xu, W. and Fu, H., J. Org. Chem., 2011, vol. 76, p. 3846. https://doi.org/10.1021/jo2002227
Liu, X., Fu, H., Jiang, Y., and Zhao, Y., Angew. Chem., Int. Ed., 2009, vol. 121, p. 354. https://doi.org/10.1002/ange.200804675
Kumar, D., Jadhavar, P.S., Nautiyal, M., Sharma, H., Meena, P.K., Adane, L., Pancholia, S., and Chakraborti, A.K., RSC Adv., 2015, vol. 5, p. 30819. https://doi.org/10.1039/C5RA03888J
Hisano, T., Ichikawa, M., Nakagawa, A., and Tsuji, N.M., Chem. Pharm. Bull., 1975, vol. 23, p. 1910. https://doi.org/10.1248/cpb.23.1910
Abdel-Jalil, R.J., Voelter, W., and Saeed, M., Tetrahedron Lett., 2004, vol. 45, p. 3475. https://doi.org/10.1016/j.tetlet.2004.03.003
Mitobe, Y., Ito, S., Mizutani, T., Nagase, T., Sato, N., and Tokita, S., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 4075. https://doi.org/10.1016/j.bmcl.2009.06.025
Balakumar, C., Lamba, D., Kishore, P., Narayana, B.L., Rao, K.V., Rajwinder, K., Rao, A.R., Shireesha, B., and Narsaiah, B., Eur. J. Med. Chem., 2010, vol. 45, p. 4904. https://doi.org/10.1016/j.ejmech.2010.07.063
Juvale, K. and Wiese, M., Bioorg. Med. Chem. Lett., 2012, vol. 22, p. 6766. https://doi.org/10.1016/j.bmcl.2012.08.024
Cheng, R., Guo, T., Zhang-Negrerie, D., Du, Y., and Zhao, K., Synthesis, 2013, vol. 45, p. 2998. https://doi.org/10.1055/s-0033-1338521
Parashuram, L., Sreenivasa, S., Akshatha, S., Kumar, V.U., and Kumar, S., Asian J. Org. Chem., 2017, vol. 6, p. 1755. https://doi.org/10.1002/ajoc.201700467
Sharif, M., Opalach, J., Langer, P., Beller, M., and Wu, X., RSC Adv., 2014, vol. 4, p. 8. https://doi.org/10.1039/C3RA45765F
Hu, B-Q., Cui, J., Wang, L-X., and Yang, L., RSC Adv., 2016, vol. 6, p. 43950. https://doi.org/10.1039/C6RA05777B
Banerjee, B., J. Nanochem., 2017, vol. 7, p. 389. https://doi.org/10.1007/s40097-017-0247-0
Bandgar, B.P., More, P.E., Kamble, V.T., and Totre, J.V., Arkivoc, 2008, vol. 2008, part (xv), p. 1. https://doi.org/10.3998/ark.5550190.0009.f01
More, P.E., Kamble, V.T., and Bandgar; B.P., Catal. Commun., 2012, vol. 27, p 30. https://doi.org/10.1016/j.catcom.2012.06.012
Bandgar, B.P., More, P.E., Kamble, V.T., and Sawant, S.S., Aust. J. Chem., 2008, vol. 61, p. 1006. https://doi.org/10.1071/CH08202
Kim, N.Y. and Cheon, C-H., Tetrahedron Lett., 2014, vol. 55, p. 2340. https://doi.org/10.1016/j.tetlet.2014.02.065
To, T.A., Vo, Y.H., Nguyen, H.T.T., Ha, P.T.M., Doan, S.H., Doan, T.L.H., Li, S., Le, H.V., Tu, T.N., and Phan, N.T.S., J. Catal., 2019, vol. 370, p. 11. https://doi.org/10.1016/j.jcat.2018.11.031
Zhou, J. and Fang, J., J. Org. Chem., 2011, vol. 76, p. 7730. https://doi.org/10.1021/jo201054k
Parua, S., Das, S., Sikari, S., Sinha, S., and Paul, N., J. Org. Chem., 2017, vol. 82, p. 7165. https://doi.org/10.1021/acs.joc.7b00643
ACKNOWLEDGMENTS
The authors are thankful to Chairman, Agricultural Development Trust Baramati, Dist. Pune, for providing all necessary facilities required for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
More, P.E., Khillare, S.L., Shinde, N.S. et al. Solvent-Free Synthesis of Quinazolinone Derivatives Catalyzed by Wet Zinc Oxide Nanopowder under Air Atmosphere. Russ J Org Chem 58, 119–124 (2022). https://doi.org/10.1134/S1070428022010171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022010171