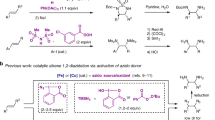
Abstract
Electrochemical synthesis of gem-bis(sulfenyl)enamines from vinyl azides and thiols is performed. The reaction occurs in an undivided electrochemical cell equipped with platinum electrodes in a DMSO–MeCN solution at a current density of 20 mA/cm2, using NH4I as a supporting electrolyte and a redox catalyst. The yields of the target products are 33–58%.


Similar content being viewed by others
REFERENCES
Arnone, A., Cardillo, R., Di Modugno, V., and Nasini, G., J. Chem. Soc. Perkin Trans. 1, 1989, p. 1995. https://doi.org/10.1039/p19890001995
Stallard, M.O., Fenical, W., and Kittredge, J.S., Tetrahedron, 1978, vol. 34, p. 2077. https://doi.org/10.1016/0040-4020(78)89006-1
Scott, L.J. and Lamb, H.M., Drugs, 1999, vol. 58, p. 499. https://doi.org/10.2165/00003495-199958030-00016
Buckley, M.M. and Goa, K.L., Drugs, 1989, vol. 37, p. 451. https://doi.org/10.2165/00003495-198937040-00004
Chelucci, G., Chem. Rev., 2012, vol. 112, p. 1344. https://doi.org/10.1021/cr200165q
Pawluć, P., Hreczycho, G., Suchecki, A., Kubicki, M., and Marciniec, B., Tetrahedron, 2009, vol. 65, p. 5497. https://doi.org/10.1016/j.tet.2009.01.113
Zhang, X., Liu, C., Deng, Y., and Cao, S., Org. Biomol. Chem., 2020, vol. 18, p. 7540. https://doi.org/10.1039/d0ob01821j
Yoo, W.J., Kondo, J., Rodriguez-Santamaria, J.A., Nguyen, T.V.Q., and Kobayashi, S., Angew. Chem. Int. Ed., 2019, vol. 58, p. 6772. https://doi.org/10.1002/anie.201902779
Kumar, N., Eghbarieh, N., Stein, T., Shames, A.I., and Masarwa, A., Chem. Eur. J., 2020, vol. 26, p. 5360. https://doi.org/10.1002/chem.202000603
Zubkov, M.O., Kosobokov, M.D., Levin, V.V., Kokorekin, V.A., Korlyukov, A.A., Hu, J., and Dilman, A.D., Chem. Sci., 2020, vol. 11, p. 737. https://doi.org/10.1039/c9sc04643g
Fu, J., Zanoni, G., Anderson, E.A., and Bi, X., Chem. Soc. Rev., 2017, vol. 46, p. 7208. https://doi.org/10.1039/c7cs00017k
Wang, Y.F., Lonca, G.H., and Chiba, S., Angew. Chem. Int. Ed., 2014, vol. 53, p. 1067. https://doi.org/10.1002/anie.201307846
Paveliev, S.A., Churakov, A.I., Alimkhanova, L.S., Segida, O.O., Nikishin, G.I., and Terent’ev, A.O., Ad. Synth. Catal., 2020, vol. 362, p. 3864. https://doi.org/10.1002/adsc.202000618
Mao, L.L., Quan, L.X., Zhu, X.H., Ji, C.B., Zhou, A.X., Chen, F.Y., and Zheng, D.G., Synlett, 2019, vol. 30, p. 955. https://doi.org/10.1055/s-0037-1611758
Li, G., Kong, X., Liang, Q., Lin, L., Yu, K., Xu, B., and Chen, Q., Eur. J. Org. Chem., 2020, 2020, p. 6135. https://doi.org/10.1002/ejoc.202001059
Ning, Y., Zhao, X.F., Wu, Y.B., and Bi, X., Org. Lett., 2017, vol. 19, p. 6240. https://doi.org/10.1021/acs.orglett.7b03204
Mulina, O.M., Zhironkina, N.V., Paveliev, S.A., Demchuk, D.V., and Terent’ev, A.O., Org. Lett., 2020, vol. 22, p. 1818. https://doi.org/10.1021/acs.orglett.0c00139
Mulina, O.M., Ilovaisky, A.I., Opatz, T., and Terent’ev, A.O., Tetrahedron Lett., 2021, vol. 64, p. 152737. https://doi.org/10.1016/j.tetlet.2020.152737
Montevecchi, P.C., Navacchia, M.L., and Spagnolo, P., J. Org. Chem., 1997, vol. 62, p. 5846. https://doi.org/10.1021/jo970691q
Terent’ev, A.O., Mulina, O.M., Ilovaisky, A.I., Kokorekin, V.A., and Nikishin, G.I., Mendeleev Commun., 2019, vol. 29, p. 80. https://doi.org/10.1016/j.mencom.2019.01.027
Liu, K., Song, C., and Lei, A., Org. Biomol. Chem., 2018, vol. 16, p. 2375. https://doi.org/10.1039/C8OB00063H
Tang, H.T., Jia, J.S., and Pan, Y.M., Org. Biomol. Chem., 2020, vol. 18, p. 5315. https://doi.org/10.1039/d0ob01008a
Martins, G.M., Meirinho, A.G., Ahmed, N., Braga, A.L., and Mendes, S.R., ChemElectroChem., 2019, vol. 6, p. 5928. https://doi.org/10.1002/celc.201901525
Pramanik, M., Choudhuri, K., and Mal, P., Org. Biomol. Chem., 2020, vol. 18, p. 8771. https://doi.org/10.1039/d0ob01741h
Hayashi, H., Kaga, A., and Chiba, S., J. Org. Chem., 2017, vol. 82, p. 11981. https://doi.org/10.1021/acs.joc.7b02455
Ni, J., Mao, X., and, Zhang, A., Adv. Synth. Catal., 2019, vol. 361, p. 2004. https://doi.org/10.1002/adsc.201900035
Funding
The work was financially supported by the Russian Foundation for Basic Research (project no. 19-29-08027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 8, pp. 1185–1192 https://doi.org/10.31857/S0514749221080103.
Rights and permissions
About this article
Cite this article
Mulina, O.M., Doronin, M.M., Kostyagina, V.A. et al. Electrochemical Synthesis of gem-Bis(sulfenyl)enamines from Vinyl Azides and Thiols. Russ J Org Chem 57, 1302–1308 (2021). https://doi.org/10.1134/S1070428021080108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021080108