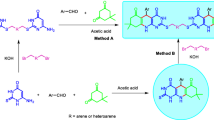
Abstract
Reactions of (chlorosulfanyl)benzenes with benzene-1,4-diamines afforded new N,N′-bis(arylsulfanyl)cyclohexa-2,5-diene-1,4-diimines which were oxidized to N,N′-(cyclohexa-2,5-diene-1,4-diylidene)bis(arenesulfinamides) with 2 equiv of m-chloroperoxybenzoic acid. When the oxidation was carried out using 4 equiv of m-chloroperoxybenzoic acid, the corresponding bis-sulfonamides were obtained. The synthesized compounds were predicted in silico to exhibit biological activity with a high probability.






Similar content being viewed by others
REFERENCES
Abraham, I., Joshi, R., Pardasani, P., and Pardasani, R.T., J. Braz. Chem. Soc., 2011, vol. 22, p. 385. https://doi.org/10.1590/S0103-50532011000300002
Avdeenko, A.P. and Konovalova, S.A., Khinoniminy: ot protivorakovykh preparatov do molekulyarnykh komp’yuterov (Quinone Imines: From Anticancer Drugs to Molecular Computers), Kramatorsk: Donbass Gos. Mashinostr. Akad., 2018, p. 238.
Kacmaz, A., J. Turk. Chem. Soc. A, 2018, vol. 5, p. 963. https://doi.org/10.18596/jotcsa.429197
Dandawate, P.R., Vyas, A.C., Padhye, S.B., Singh, M.W., and Baruah, J.B., Mini-Rev. Med. Chem., 2010, vol. 10, p. 436. https://doi.org/10.2174/138955710791330909
Breyer, S., Effenberger, K., and Schobert, R., ChemMedChem, 2009, vol. 4, p. 761. https://doi.org/10.1002/cmdc.200800430
Petronzi, S., Festa, M., Peduto, A., Castellano, M., Marinello, J., Massa, A., Capasso, A., Capranico, G., La Gatta, A., De Rosa, M., Caraglia, M., and Filosa, R., J. Exp. Clin. Cancer Res., 2013, vol. 32, p. 24. https://doi.org/10.1186/1756-9966-32-24
Delarmelina, M., Daltoé, R.D., Cerri, M.F., Madeira, K.P., Rangel, L.B.A., Lacerda Júnior, V., Romão, W., Taranto, A.G., and Greco, S.J., J. Braz. Chem. Soc., 2015, vol. 26, p. 1804. https://doi.org/10.5935/0103-5053.20150157
Burke, A.S., MacMillan-Crow, L.A., and Hinson, J.A., Chem. Res. Toxicol., 2010, vol. 23, p. 1855. https://doi.org/10.1021/tx1003744
Bolton, J.L. and Dunlap, T., Chem. Res. Toxicol., 2017, vol. 30, p. 13. https://doi.org/10.1021/acs.chemrestox.6b00256
Vasylyuk, S., Komarovska-Porokhnyavets, O., Novikov, V., and Lubenets, V., Chem. Chem. Technol., 2018, vol. 12, p. 24. https://doi.org/10.23939/chcht12.01.024
Oriabinska, L.B., Starovoitova, S.O., Vasylyuk, S.V., Novikov, V.P., and Lubenets, V.I., Ukr. Biochem. J., 2017, vol. 89, p. 70. https://doi.org/10.15407/ubj89.05.070
Povarov, I.G., Shilenkov, N.A., Krasnov, P.O., Suboch, G.A., and Tovbis, M.S., Russ. J. Org. Chem., 2020, vol. 56, p. 1412. https://doi.org/10.1134/S1070428020080126
Kuz’menko, L., Avdeenko, A., Konovalova, S., Vasylyuk, S., Fedorova, O., Monka, N., Krychkovska, A., and Lubenets, V., Biointerface Res. Appl. Chem., 2019, vol. 9, p. 4232. https://doi.org/10.33263/BRIAC95.232238
Greenhalgh, C.W., Shand, C.A., and Thomson, R.H., J. Chem. Res., Synop., 1982, no. 6, p. 138. https://doi.org/10.1002/chin.198238177
Avdeenko, A.P., Pirozhenko, V.V., Stanovskii, M.V., Konovalova, S.A., and Yusina, A.L., Russ. J. Org. Chem., 2004, vol. 40, p. 1291. https://doi.org/10.1007/s11178-005-0008-2
Avdeenko, A.R., Pirozhenko, V.V., Konovalova, S.A., Santalova, A.A., and Vakulenko, A.V., Arkivoc, 2005, vol. 2005, part (viii), p. 60. https://doi.org/10.3998/ark.5550190.0006.805
Ibis, C., Sahinler Ayla, S., Tulegenova, D., and Bahar, H., Russ. J. Org. Chem., 2019, vol. 55, p. 546. https://doi.org/10.1134/S1070428019040213
Sergeev, V.A., Nedel’kin, V.N., Arnautov, S.A., and Prokof’ev, A.I., Bull. Acad. Sci. USSR, Div. Chem. Sci., 1984, vol. 33, p. 1957. https://doi.org/10.1007/BF00948654
Pirozhenko, V.V., Rozhenko, A.B., Avdeenko, A.P., Konovalova, S.A., and Santalova, A.A., Magn. Reson. Chem., 2008, vol. 46, p. 811. https://doi.org/10.1002/mrc.2254
Avdeenko, A.P., Konovalova, S.A., and Santalova, A.A., Russ. J. Org. Chem., 2008, vol. 44, p. 231. https://doi.org/10.1007/s11178-008-2008-5
Filimonov, D.A., Lagunin, A.A., Gloriozova, T.A., Rudik, A.V., Druzhilovskii, D.S., Pogodin, P.V., and Poroikov, V.V., Chem. Heterocycl. Compd., 2014, vol. 50, p. 444. https://doi.org/10.1007/s10593-014-1496-1
Mueller, W.H. and Butler, P.E., J. Org. Chem., 1968, vol. 33, p. 1533. https://doi.org/10.1021/jo01268a049
Konovalova, S.A., Avdeenko, A.P., Santalova, A.A., Palamarchuk, G.V., D’yakonenko, V.V., and Shishkin, O.V., Russ. J. Org. Chem., 2015, vol. 51, p. 42. https://doi.org/10.1134/S1070428015010078
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 4, pp. 532–540 https://doi.org/10.31857/S051474922104008X.
Rights and permissions
About this article
Cite this article
Konovalova, S.A., Avdeenko, A.P. & Santalova, A.A. Synthesis of N,N′-Bis(arylsulfanyl)cyclohexa-2,5-diene-1,4-diimines and N,N′-(Cyclohexa-2,5-diene-1,4-diylidene)bis(arenesulfinamides). Russ J Org Chem 57, 551–557 (2021). https://doi.org/10.1134/S1070428021040084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021040084