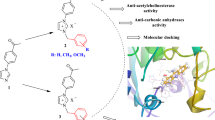
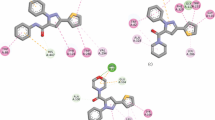
Abstract
New pyridine-2,6-dicarboxamide derivatives containing sulfonamide groups were synthesized by the coupling of pyridine-2,6-dicarbonyl dichloride and various aminobenzenesulfonamides in a mixture of dichloromethane and acetone. The pyridinedicarboxamide-based sulfonamides were evaluated as carbonic anhydrase (CA) and cholinesterase (ChE) inhibitors, and they showed IC50 values in the ranges 12.8–37.6 nM against human carbonic anhydrase I (hCA I), 17.8–46.7 nM against human carbonic anhydrase II (hCA II), 98.4–197.5 nM against acetylcholinesterase (AChE), and 82.2–172.7 nM against butyrylcholinesterase (BuChE). These results are comparable with those for known inhibitors such as acetazolamide (IC50 = 32.1 nM for hCA I and IC50 = 51.0 nM for hCA II) and rivastigmine (IC50 = 60.2 nM for AChE and IC50 = 14.0 nM for BuChE), which qualifies the synthesized compounds as candidates for a more in-depth study.
Similar content being viewed by others
References
Comprehensive Heterocyclic Chemistry III, Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., and Taylor, R.J.K., Eds., Amsterdam: Elsevier, 2008.
Perez-Mayoral, E., Calvino-Casilda, V., Godino, M., Lopez-Peinado, A.J., and Martin-Aranda, R.M., Green Synthetic Approaches for Biologically Relevant Heterocycles, Brahmachari, G., Ed., Waltham, MA: Elsevier, 2014. chap. 15, p. 377. https://doi.org/10.1016/B978-0-12-800070-0.00015-3
El-Sayed, H.A., Moustafa, A.H., El-Torky, A.E., and El-Salam, E.A.A., Russ. J. Gen. Chem., 2017, vol. 87, p. 2401. https://doi.org/10.1134/S107036321710022X
Kumar, P. and Gupta, R., Dalton Trans., 2016, vol. 45, p. 18769. https://doi.org/10.1039/C6DT03578G
Gudasi, K.B., Patil, S.A., Vadavi, R.S., Shenoy, R.V., and Patil, M.S., J. Serb. Chem. Soc., 2006, vol. 71, p. 529. https://doi.org/10.2298/JSC0605529G
Lu, X., Zhang, H., Li, X., Chen, G., Li, Q.S., Luo, Y., Ruan, B.F., Chen, X.W., and Zhu, H.L., Bioorg. Med. Chem., 2011, vol. 19, p. 6827. https://doi.org/10.1016/j.bmc.2011.09.034
Abo-Ghalia, M. and Amr, A., Amino Acids, 2004, vol. 26, p. 283. https://doi.org/10.1007/s00726-003-0042-8
Amr, A.E.G.E., Mohamed, A.M., and Ibrahim, A.A., Z. Naturforsch., Teil B, 2003, vol. 58, p. 861.
Amr, A.E.G.E., Abo-Ghalia, M., and Abdalah, M.M., Z. Naturforsch., Teil B, 2006, vol. 61, p. 1335.
Al-Salahi, R.A., Al-Omar, M.A., and Amr, A.E.G.E., Molecules, 2010, vol. 15, p. 6588. https://doi.org/10.3390/molecules15096588
Abo-Ghalia, M.H., Amr, A.E.G.E., and Abdalah, M.M., Z. Naturforsch., Teil B, 2003, vol. 58, p. 903.
Riaz, S., Khan, I.U., Bajda, M., Ashraf, M., Ain, Q., Shaukat, A., Rehman, T.U., Mutahir, S., Hussain, S., Mustafa, G., and Yar, M., Bioorg. Chem., 2015, vol. 63, p. 64. https://doi.org/10.1016/j.bioorg.2015.09.008
Supuran, C.T., Scozzafava, A., and Casini, A., Med. Res. Rev., 2003, vol. 23, p. 146. https://doi.org/10.1002/med.10025
Supuran, C.T., Nat. Rev. Drug Discovery, 2008, vol. 7, p. 168. https://doi.org/10.1038/nrd2467
Pichake, J., Kharkar, P.S., Ceruso, M., Supuran, C.T., and Toraskar, M.P., ACS Med. Chem. Lett., 2014, vol. 5, p. 793. https://doi.org/10.1021/ml500140t
Scozzafava, A., Mastrolorenzo, A., and Supuran, C.T., Expert Opin. Ther. Pat., 2004, vol. 14, p. 667. https://doi.org/10.1517/13543776.14.5.667
Supuran, C.T., Therapy, 2007, vol. 4, p. 355. https://doi.org/10.2217/14750708.4.3.355
Maren, T.H. and Conroy, C.W., J. Biol. Chem., 1993, vol. 268, p. 26233. https://doi.org/10.1039/B417327A
Sapegin, A., Kalinin, S., Angeli, A., Supuran, C.T., and Krasavin, M., Bioog. Chem., 2018, vol. 76, p. 140. https://doi.org/10.1016/j.bioorg.2017.11.014
Stellenboom, N., J. Biochem. Mol. Toxicol., 2019, vol. 33, article no. e22240. https://doi.org/10.1002/jbt.22240
Verpoorte, J.A., Mehta, S., and Edsall, J.T., J. Biol. Chem., 1967, vol. 242, p. 4221.
Cheng, Y.C. and Prusoff, W.H., Biochem. Pharmacol., 1973, vol. 22, p. 3099. https://doi.org/10.1016/0006-2952(73)90196-2
Ellman, G.L., Courtney, K.D., Andres, V., and Featherstone, R.M., Biochem. Pharmacol., 1961, vol. 7, p. 88. https://doi.org/10.1016/0006-2952(61)90145-9
Acknowledgments
The authors thank Agri Ibrahim Cecen University for providing facilities for carrying out this research and Prof. R. Hunter for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
No conflict of interest is declared by the authors.
Rights and permissions
About this article
Cite this article
Stellenboom, N., Baykan, A.R. Synthesis and Enzyme Inhibitory Activity of Novel Pyridine-2,6-dicarboxamides Bearing Primary Sulfonamide Groups. Russ J Org Chem 55, 1951–1956 (2019). https://doi.org/10.1134/S1070428019120248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019120248