Abstract
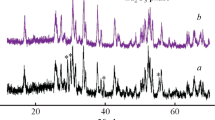
Li7La3Zr2O12-based compounds are today the most promising solid electrolytes for high-energy lithium and lithium–ion power sources. The solid electrolytes Li7–x–3yAlyLa3Zr2−xTaxO12 (x = 0.3–0.6, y = 0.05–0.20) were prepared by the sol–gel method. The effect of doping of Li7La3Zr2O12 in Zr and Li sublattices with tantalum (Ta5+) and aluminum (Al3+) on the crystal structure, morphology, and electrical conductivity of this compound was examined. The compounds obtained had the cubic structure (space group Ia-3d). The resistance of the solid electrolytes obtained was determined by the electrochemical impedance method. The compounds Li6.25Al0.15La3Zr1.7Ta0.3O12, Li6.3Al0.10La3Zr1.6Ta0.4O12, Li6.2Al0.10La3Zr1.5Ta0.5O12, and Li6.25Al0.05La3Zr1.4Ta0.6O12 have the maximal lithium-ion conductivity (~2.0 × 10–4 S cm–1 at 20°C). The heat treatment at 1150°C for 1 h is optimum for forming highly conducting and dense ceramic membranes. Symmetrical cells with Li electrodes show stable behavior in cycling. The solid electrolytes obtained can be used in lithium power sources.






Similar content being viewed by others
REFERENCES
Tarascon, J.M. and Armand, M., Nature, 2001, vol. 414, pp. 359–367. https://doi.org/10.1038/35104644
Yaroslavtsev, A.B., Russ. Chem. Rev., 2016, vol. 85, pp. 1255–1276. http://iopscience.iop.org/article/10.1070/RCR4634/pdf.
Bensalah, N. and Dawood, H., Mater. Sci. Eng., 2016, vol. 5, pp. 1–21. https://doi.org/10.4172/2169-0022.1000258
Lu, J., Chen, Z., Pan, F., Cui, Y., and Amine, K., Electrochem. Energy Rev., 2018, vol. 1, pp. 35–53. https://doi.org/10.1007/s41918-018-0001-453
Murugan, R., Thangadurai, V., and Weppner, W., Angew. Chem. Int. Ed., 2007, vol. 46, pp. 7778–7781. https://doi.org/10.1002/anie.200701144
Ramakumar, S., Deviannapoorani, C., Dhivya, L., Shankar, L.S., and Murugan, R., Prog. Mater. Sci., 2017, vol. 88, pp. 325–411. https://doi.org/10.1016/j.pmatsci.2017.04.007
Dermenci, K.B., Cekic, E., and Turan, S., Int. J. Hydrogen Energy, 2016, vol. 41, pp. 9860–9867. https://doi.org/10.1016/j.ijhydene.2016.03.197
Dermenci, K.B. and Turan, S., Ionics, 2020, vol. 26, pp. 4757–4762. https://doi.org/10.1007/s11581-020-03685-4
Zhao, P., Xiang, Y., Wen, Y., Li, M., Zhu, X., Zhao, S., Jin, Z., Ming, H., and Cao, G., J. Eur. Ceram. Soc., 2018, vol. 38, pp. 5454–5462. https://doi.org/10.1016/j.jeurceramsoc.2018.08.037
Zhao, P., Cao, G., Jin, Z., Ming, H., Wen, Y., Xu, Y., Zhu, X., Xiang, Y., and Zhang, S., Mater. Des., 2018, vol. 139, pp. 65–71. https://doi.org/10.1016/j.matdes.2017.10.067
Li, Y., Wang, C.A., Xie, H., Cheng, J., and Goodenough, J.B., Electrochem. Commun., 2011, vol. 13, pp. 1289–1292. https://doi.org/10.1016/j.elecom.2011.07.008
Wang, Y. and Lai, W., Electrochem. Solid-State Lett., 2012, vol. 5, pp. A68–A71. https://doi.org/10.1149/2.024205esl
Gong, Y., Liu, Z.G., Jin, Y.J., Ouyang, J.H., Chen, L., and Wang, Y.J., Ceram. Int., 2019, vol. 45, pp. 18439–18444. https://doi.org/10.1016/j.ceramint.2019.06.061
Janani, N., Ramakumar, S., Kannan, S., and Murugan, R., J. Am. Ceram. Soc., 2015, vol. 98, pp. 2039–2046. https://doi.org/10.1111/jace.13578
Tsai, C.-L., Roddatis, V., Chandran, C.V., Ma, Q., Uhlenbruck, S., Bram, M., and Guillon, O., ACS Appl. Mater. Interfaces, 2016, vol. 8, pp. 10617–10626. https://doi.org/10.1021/acsami.6b00831
Buschmann, H., Berendts, S., Mogwitz, B., and Janek, J., J. Power Sources, 2012, vol. 206, pp. 236–244. https://doi.org/10.1016/j.jpowsour.2012.01.094
Huang, M., Shoji, M., Shen, Y., Nan, C.W., Munakata, H., and Kanamura, K., J. Power Sources, 2014, vol. 261, pp. 206–211. https://doi.org/10.1016/j.jpowsour.2014.03.070
Li, Y., Han, J.T., Wang, C.A., Xie, H., and Goodenough, J.B., J. Mater. Chem., 2012, vol. 22, pp. 15357–15361. https://doi.org/10.1039/c2jm31413d
Il’ina, E.A., Andreev, O.L., Antonov, B.D., and Batalov, N.N., J. Power Sources, 2012, vol. 201, pp. 169–173. https://doi.org/10.1016/j.jpowsour.2011.10.108
Il’ina, E.A., Lyalin, E.D., Antonov, B.D., and Pankratov, A.A., Russ. J. Appl. Chem., 2019, vol. 92, no. 12, pp. 1657−1663. https://doi.org/10.1134/S107042721912005X
Il’ina, E.A., Lyalin, E.D., Antonov, B.D., Pankratov, A.A., and Vovkotrub, E.G., Ionics, 2020, vol. 26, pp. 3239–3247. https://doi.org/10.1007/s11581-020-03492-x
Xie, H., Alonso, J.A., Li, Y., Fernandez-Diaz, M.T., and Goodenough, J.B., Chem. Mater., 2011, vol. 23, pp. 3587–3589. https://doi.org/10.1021/cm201671k
ACKNOWLEDGMENTS
The research has been carried out with the equipment of the Shared Access Center “Composition of Compounds” of the Institute of High Temperature Electrochemistry, Ural Branch, Russian Academy of Sciences.
Funding
The synthesis of the solid electrolytes and study of their composition, morphology, and electrical conductivity were financially supported by the Russian Foundation for Basic Research and Sverdlovsk oblast, project no. 20-43-660015. The behavior of the symmetric cell with Li was studied within the framework of state budget themes of the Institute of High-Temperature Electrochemistry, Ural Branch, Russian Academy of Sciences (research program no. 122020100210-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 5, pp. 627–635, May, 2022 https://doi.org/10.31857/S0044461822050097
Rights and permissions
About this article
Cite this article
Il’ina, E.A., Lyalin, E.D., Antonov, B.D. et al. Li7La3Zr2O12-Based Solid Electrolytes Codoped with Ta5+ and Al3+ Ions for Lithium Power Sources. Russ J Appl Chem 95, 689–697 (2022). https://doi.org/10.1134/S1070427222050093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222050093