Abstract
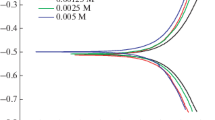
Corrosion inhibition of phenylalanine (phe) and its its cobalt complex(Co-phe) were evaluated using electrochemical polarization and electrochemical impedance spectroscopy (EIS) for mild steel in 0.1 M H2SO4 solution. The results indicate that the compounds inhibit the corrosion of mild steel in H2SO4 solutions and the extent of inhibition increases with inhibitor concentration and decreases with temperature. The order of inhibition efficiency obtained was Co-phe > phe. The adsorption characteristics of the inhibitor were approximated by Langmuir isotherm. Morphological study of the carbon steel electrode surface was undertaken by scanning electron microscope (SEM).
Similar content being viewed by others
References
Bouklah, M., Ouassini, A., Hammouti, B. and El Idrissi A., Appl. Surf. Sci., 2006, vol. 252, pp. 2178–2185.
Caliskan, N., and Akbas, E. Mater. Chem. Phys., 2011, vol. 126, pp. 983–988.
Awad, H.S., and Gawad, S.A. Anti Corros. Meth. Mater., 2005, vol. 52, pp. 328–336.
Chetouani, A., Medjahed, K., Sid-Lakhdar, K.E., Hammouti, B., Benkaddour, M. and Mansri, A. Corros. Sci., 2004, vol. 46, pp. 2421–2430.
Ergun, Umit, Yuzer, Devrim, and Emregul, Kaan C., Mater. Chem. Phys., 2008, vol. 109, pp. 492–499.
Khaled, K.F., Corros. Sci., 2010, 52, pp. 3225–3234.
Da-Quan Zhang, Bin Xie, Li-Xin Gao, Qi-Rui Cai, Hyung Goun Joo and Kang Yong Lee, Thin Solid Film, 2011, vol. 520, pp. 356–361.
Bereket, G. and Yurt, A., Corros. Sci., 2001, vol. 43, pp. 1179–1195.
Barouni, K., Bazzi, L., Salghi, R., Mihit, M., Hammouti, B., Albourine, A., and El Issami, S., Mater. Lett., 2008, vol. 62, pp. 3325–3327.
Ashassi-Sorkhabi, H., Majidi, M.R. and Seyyedi, K., Appl. Surf. Sci., 2004, vol. 225, pp. 176–185.
Ashassi-Sorkhabi, H., Ghasemi, Z., and Seifzadeh, D., Appl. Surf. Sci., 2005, vol. 249, pp. 408–418.
Gokhan Gece and Semra Bilgic, Corros. Sci., 2010, vol. 52, pp. 3435–3443.
Da-Quan Zhang, Qi-Rui Cai, Xian-Ming He, Li-Xin Gao, and Guo-Ding Zhou, Mater. Chem. Phys., 2008, vol. 112, pp. 353–358.
Ameer, M.A. and Fekry, A.M., Int. J. Hydrogen Energy, 2010, vol. 35, pp. 11387–11396.
Mahdavian, M. And Attar, M.M., Corros. Sci., 2009, vol. 51, pp. 409–414.
Khaled, K.F., Babic-Samardzija, K. and Hackerman, N., Corros. Sci., 2006, vol. 48, pp. 3014–3034.
Sha Cheng, Chen, Sh., Liu, T., et al., Electrochim. Acta, 2007, vol. 52, pp. 5932–5938.
Abdallah, M., Meghed, H. E., and Sobhi, M., Mater. Chem. Phys., 2009, vol. 118, pp. 111–117.
Alshima’, A., Massoud, Ahmed, Hefnawy, Vratislav, Langer, Mohamed, A. Khatab, Lars Öhrstrom, and Morsy, A.M. Abu-Youssef, Polyhedron, 2009, vol. 28, pp. 2794–2802.
Amar, H., Benzakour, J., Derja, A., Villemin, Moreau, D.B. Braisaz, T., and Tounsi, A., Corros. Sci., (2008) vol. 50, pp. 124–130.
Aytac, A., J. Mater. Sci., 2010, vol. 45, pp. 6812–6818.
Singh, V. P., Singh, P., and Singh, A. K., Inorg. Chimica Acta, 2011, vol. 379, pp. 56–63.
Singh, P., Singh, A.K., and Singh, V.P., Polyhedron, 2013, vol. 65, pp. 73–81.
Liu, X., Okafor, P.C. and Zheng, Y.G.., Corros. Sci., 2009, vol. 51, pp. 744–751.
Bahrami, M.J., Hosseini, S.M.A., and Pilvar, P., Corros. Sci., 2010, vol. 52, pp. 2793–2803.
Benabdellah, M., Touzani, R., Dafali, A., Hammouti, B., and El Kadiri, S., Mater. Lett., 2007, vol. 61, pp. 1197–1204.
Gomma, K. and Wahdan, M.H., Mater. Chem. Phys. 1994, vol. 32, pp. 142–148.
Martinez, S. and Stern, I., Appl. Surf. Sci., 2002, vol. 199, pp. 83–89.
Noor, E. A. and Al-Moubaraki. Mater. Chem. Phys., 2008, vol. 110, pp. 145–154.
Xiumei Wang, Huaiyu Yang and Fuhui Wang, Corros. Sci., 2010, vol. 52, pp. 1268–1276.
Refaey, S.A.M., Taha, F., and Abd El-Malak, A.M., Appl. Surf. Sci., 2004, vol. 236, pp. 175–185.
Ashassi-Sorkhabi, H., Shaabani, B., and Seifzadeha, D., Electrochim. Acta, 2005, vol. 50, pp. 3446–3452.
Obot, I.B., Obi-Egbedi, N.O., and Odozi, N.W., Corros. Sci., 2010, vol. 52, pp. 923–926.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Liu, X., Lu, Y., Yang, Y.S. et al. Electrochemical behavior of phenylalanine and its cobalt complex as corrosion inhibitors for mild steel in H2SO4 . Russ J Appl Chem 88, 350–355 (2015). https://doi.org/10.1134/S1070427215020263
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427215020263