Abstract
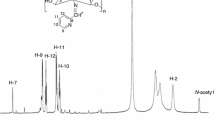
Schiff derivatives were prepared by the reactions of salicylaldehyde and its derivatives (5-chloro, 5-methoxy, 5-fluoro, 5-methyl, 5-nitro) with the amino group of chitosan. The Schiff bases were studied by Fourier IR spectroscopy and by UV-visible spectroscopy. The cyclic voltammograms of the Schiff bases were analyzed and compared to those of chitosan and salicylaldehyde. The formal potential of the chitosan Schiff base derivative correlates with the Hammett parameters. The oxidation potential increases and the optical density decreases with enhancement of the electron-acceptor properties of the functional group R in the m-position to the -N=CH-group. Chitosan (Chi) is a polysaccharide whose chains consist of recurrent units of acetamido-2-deoxy-D-glucode linked by the 1,4-β-glycoside bond. This polysaccharide was widely studied as drug carrier [1, 2], because it is nontoxic, biodegradable, and well biocompatible [3].
Similar content being viewed by others
References
Nagpal, K., Singh, S.K., and Mishra, D.N., Chem. Pharm. Bull., 2010, vol. 58, no. 11, p. 1423.
Wang, J.J., Zeng, Z.W., Xiao, R.Z., et al., Int. J. Nanomed., 2011, vol. 6, p. 765.
Peniche, C., Arguelles-Monal, W., Peniche, H., and Acosta, N., Macromol. Biosci., 2003, vol. 3, p. 511.
Sajomsang, W., Tantayanon, S., Tangpasuthadol, V., and Daly, W.H., Carbohydr. Res., 2009, vol. 344, p. 2502.
Neveen, A.A., Shawky, M.H., Eman, M.S., et al., Carbohydr. Res., 2011, vol. 346, p. 775.
Monier, M., Ayad, D.M., Wei, Y., and Sarhan, A.A., J. Hazard. Mater., 2010, vol. 177, p. 962.
Ying, G., Xiong, W., Wang, H., et al., Carbohydr. Polym., 2011, vol. 83, p. 1787.
Jin, X., Wang, J., and Bai, J., Carbohydr. Res., 2009, vol. 344, p. 825.
Guo, Z., Xing, R., Liu, S., et al., Bioorg. Med. Chem. Lett., 2005, vol. 15, p. 4600.
Da Silva, C.M., da Silva, D.L., Modolo, L.V., et al., J. Adv. Res., 2011, vol. 2, p. 1.
Dos Santos, J.E., Dockal, E.R., and Cavalheiro, E.T.G., Carbohydr. Polym., 2005, vol. 60, p. 277.
Shangguan, X.D. and Zheng, J.B., Electroanalysis, 2009, vol. 21, p. 881.
Hu, D.D., Shi, Q.Z., Tang, Z.X., et al., Carbohydr. Polym., 2001, vol. 45, no. 4, p. 385.
Feng, J., Zhao, G., Xu, J.-J., and Chen, H.Y., Anal. Biochem., 2005, vol. 342, p. 280.
Xu, Q., Mao, Ch., Liu, N.N., et al., Biosens. Bioelectron., 2006, vol. 22, p. 768.
Huang, H., Hu, N., Zeng, Y., and Zhou, G., Anal. Biochem., 2002, vol. 308, p. 141.
Jiang, L., Wang, R., Li, X., et al., Electrochem. Commun., 2005, vol. 7, p. 597.
Dong, S., Li, Z., Yu, Z., et al., Colloids Surf. B, 2012, vol. 100, p. 133.
Zolezzia, S., Spodinea, E., and Decintia, A., Polyhedron, 2002, vol. 21, no. 1, p. 55.
Gulppi, M.A., Paez, M.A., Costamagna, J.A., et al., J. Electroanal. Chem., 2005, vol. 580, no. 1, p. 50.
Cardenas-Jiron, G.I., Gulppi, M.A., Caro, C.A., et al., Electrochim. Acta, 2001, vol. 46, nos. 20–21, p. 3227.
Zagal, J.H., Gulppi, M.A., Caro, C.A., and Cardenas-Jiron, G.I., Electrochem. Commun., 1999, vol. 1, no. 9, p. 389.
Lexa, D., Savean, J.M., Schafer, H.J., et al., J. Am. Chem. Soc., 1990, vol. 112, p. 6162.
Saveant, J.M., J. Am. Chem. Soc., 1992, vol. 114, p. 10595.
Chang, H.-P., Yao, H.-T., and Chiang, M.-T., J. Food Drug Anal., 2012, vol. 20, p. 661.
Costamagna, J., Lillo, L.E., Matsuhiro, B., et al., Carbohydr. Res., 2003, vol. 338, p. 1535.
Lillo, L.E. and Matsuhiro, B., Carbohydr. Polym., 1997, vol. 34, p. 397.
Brugnerotto, J., Lizardi, J., Goycoolea, W., et al., Polymer, 2001, vol. 42, no. 8, p. 3569.
Hu, D.D., Shi, Q.Z., Tang, Z.X., et al., Carbohydr. Polym., 2001, vol. 45, p. 385.
Kuder, J.E., Gibson, H.W., and Wychick, D., J. Org. Chem., 1975, vol. 40, no. 7, p. 875.
Geraldo, D., Linares, C., Chen, Y.Y., et al., Electrochem. Commun., 2002, vol. 4, no. 2, p. 182.
Tse, Y., Janda, P., Lam, H., et al., J. Porphyrins Phthalocyanines, 1999, vol. 1, p. 3.
Hansch, C., Leo, A., and Taft, R.W., Chem. Rev., 1991, vol. 91, p. 165.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original English Text © C.A. Caro, G. Cabello, E. Landaeta, J. Pérez, J.H. Zagal, L. Lillo, 2013, published in Zhurnal Prikladnoi Khimii, 2013, Vol. 86, No. 11, pp. 1843–1849.
Rights and permissions
About this article
Cite this article
Caro, C.A., Cabello, G., Landaeta, E. et al. Synthesis and spectroscopic and electrochemical studies of chitosan Schiff base derivatives. Russ J Appl Chem 86, 1791–1797 (2013). https://doi.org/10.1134/S1070427213110268
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427213110268