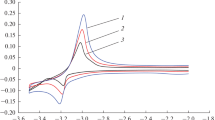
Abstract
Potentiometric method was used to measure the redox potentials of Tm3+/Tm2+ in a eutectic melt of sodium, potassium, and cesium chlorides relative to a chlorine reference electrode in the temperature range 823–973 K. The main thermodynamic characteristics of the redox reaction TmCl2(solution) + 1/2Cl2(g) ⇆ TmCl3(solution) were calculate from the conditional standard potentials \( E_{{{Tm^{3 + } } \mathord{\left/ {\vphantom {{Tm^{3 + } } {Tm^{2 + } }}} \right. \kern-\nulldelimiterspace} {Tm^{2 + } }}}^* \).
Similar content being viewed by others
References
Brown, D., Halides of the Lanthanides and Actinides, New York: Wiley, 1968.
Encyclopedia of Electrochemistry of the Elements, Bard, A.J. and Plambeck, J.A., Eds., vol. 10, Fused Salt Systems. New York: Marcel Dekker Inc., 1976.
A Lanthanide Lanthology, USA: Molycorp., Inc., 1997. Parts 1 and 2.
Kuznetsov, S.A., Hayashi, H., Minato, K., and Gaune-Escard, M., Electrochim. Acta, 2006, vol. 51, pp. 2463–2470.
Martinot, L., Handbook on the Physics and Chemistry of the Actinides, vol. 6, North Holland, Amsterdam, 1991.
The Chemistry of the Actinide and Transactinide Elements, Morss, L.R., Edelstein, N.M., and Fuger, J., Eds., vols. 1–4. Netherlands: Springer, 2008.
Qiqin, Y., Kairong, Q., Guankun, L., and Tong, G., Acta Metallurgica Sinica, 1995, vol. 31, no. 10, pp. 445–450.
Castrillejo, Y., Fernandez, P., Bermejo, M.R., et al., Electrochim. Acta, 2009, vol. 54, no. 26, pp. 6212–6222.
Novoselova, A. and Smolenski, V. J., Chem. Thermodyn., 2010, vol. 42, no. 8, pp. 973–977.
Shishkin, V.Yu. and Mityaev, V.S., Izv. Akad. Nauk SSSR, Neorg. Mater., 1982, vol. 18, no. 11, pp. 1917–1918.
Korshunov, B.G., Safonov, V.V., and Drobot, D.V., Fazovye ravnovesiya v galogenidnykh sistemakh (Phase Equilibrium in Halide Systems), Moscow: Metallurgiya, 1979.
Revzin, G.E., Metody polucheniya khimicheskikh reaktivov i preparatov (Methods for Synthesis of Chemical Reagents and Preparations), Moscow, 1967, issue 16, pp. 124–129.
Novoselova, A.V., Shishkin, V.Yu., and Khokhlov, V.A., Rasplavy, 1999, no. 6, pp. 32–39.
Kuznetsov, S.A. and Gaune-Escard, M., J. Nucl. Mater., 2009, vol. 389, no. 1, pp. 108–114.
Thompson, M. and Walsh, J,, A Handbook of Inductively Coupled Plasma Spectrometry, New York: Chapman and Hall, 1989.
Smirnov, M.V., Elektrodnye potentsialy v rasplavlennykh khloridakh (Electrode Potentials in Molten Electrolyte), Moscow: Nauka, 1973.
Lebedev, V.A., Izbiratel’nost’ zhidkometallicheskikh elektrodov v rasplavlennykh galogenidakh (Selectivity of Liquid-Metal Electrodes in Molten Halides), Chelyabinsk: Metallurgiya, 1993.
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.V. Novoselova, V.V. Smolenskii, 2010, published in Zhurnal Prikladnoi Khimii, 2010, Vol. 83, No. 11, pp. 1812–1815.
Rights and permissions
About this article
Cite this article
Novoselova, A.V., Smolenskii, V.V. Electrochemical and thermodynamic properties of thulium trichloride in a molten NaCl-KCl-CsCl eutectic. Russ J Appl Chem 83, 1944–1947 (2010). https://doi.org/10.1134/S1070427210110091
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427210110091