Abstract
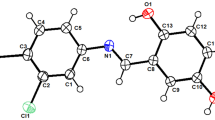
The condensation reaction of 4,6-di-tert-butyl-2,3-dihydroxybenzaldehyde with some ammonium acetohydrazides yielded water-soluble acylhydrazones with different structures of the cationic center. It was shown that in relation to Staphylococcus aureus, Bacillus cereus and Enterococcus faecalis, trimethylammonium chloride derivative exhibits activity at or above the level of comparison drugs, norfloxacin and chloramphenicol, respectively. The resulting compounds do not have a toxic effect on erythrocytes and normal human liver cells. The high activity of diethylmethylammonium acylhydrazone against the formation of biofilms formed by clinical staphylococci strains was shown for the first time. The high efficiency of new compounds in inhibiting the growth of phytopathogens of bacterial and fungal origin was established.



Similar content being viewed by others
REFERENCES
Viegas-Junior, C., Danuello, A., da Silva Bolzani, V., Barreiro, E.J., and Fraga, C.A.M., Curr. Med. Chem., 2007, vol. 14, p. 1829. https://doi.org/10.2174/092986707781058805
Berube, G., Expert Opin. Drug Discov., 2016, vol. 11, p. 281. https://doi.org/10.1517/17460441.2016.1135125
Kumar, H.M.S., Herrmann, L., and Tsogoeva, S.B., Bioorg. Med. Chem. Lett., 2020, vol. 30, p. 127514. https://doi.org/10.1016/j.bmcl.2020.127514
Design of Hybrid Molecules for Drug Development, Decker, M., Ed., Amsterdam: Elsevier, 2017.
Kataev, V.E., Strobykina, I.Yu., and Zakharova, L.Ya., Russ. Chem. Bull., 2014, vol. 63, p. 1884. https://doi.org/10.1007/s11172-014-0680-x
Osimitz, Th.G. and Droege, W., Toxicology Res. Appl., 2021, vol. 5, p. 1. https://doi.org/10.1177/23978473211049085
Padrtova, T., Marvanova, P., Odehnalova, K., Kubinova, R., Parravicini, O., Garro, A., Enriz, R.D., Humpa, O., Oravec, M., and Mokry, P., Molecules, 2017, vol. 22, p. 2048. https://doi.org/10.3390/molecules22122048
Nair, V.P. and Hunter, J.M., Continuing Educ. Anaesth. Critical Care Pain., 2004, vol. 4, p. 164. https://doi.org/10.1093/bjaceaccp/mkh045
Conejo-Garcia, A., Pisani, L., Del Carmen Nunez, M., Catto, M., Nicolotti, O., Leonetti, F., Campos, J.M., Gallo, M.A., Espinosa, A., and Carotti, A., J. Med. Chem., 2011, vol. 54, p. 2627. https://doi.org/10.1021/jm101299d
Skrzypczak, N., Pyta, K., Ruszkowski, P., Mikolajczak, P., Kucinska, M., Murias, M., Gdaniec, M., Bartl, F., and Przybylski, P., J. Enzyme Inhibit. Med. Chem., 2021, vol. 36, p. 1898. https://doi.org/10.1080/14756366.2021.1960829
Yang, J.S., Song, D., Ko, W.J., Kim, B., Kim, B.-K., Park, S.-K., Won, M., Lee, K., Lee, K., Kim, H.M., and Han, G., Eur. J. Med. Chem., 2013, vol. 63, p. 621. https://doi.org/10.1016/j.ejmech.2012.12.063
Basilico, N., Migotto, M., Ilboudo, D.P., Taramelli, D., Stradi, R., and Pini, E., Bioorg. Med. Chem., 2015, vol. 23, p. 4681. https://doi.org/10.1016/j.bmc.2015.05.055
Baker, N., Williams, A.J., Tropsha, A., and Ekins, S., Pharm. Res., 2020, vol. 37, p. 104. https://doi.org/10.1007/s11095-020-02842-8
Panunzio, M., Malabarba, A., and Vicennati, P., Arkivoc, 2004, vol. 13, p. 36. https://doi.org/10.3998/ark.5550190.0005.d05
Jennings, M.C., Minbiole, K.P.C., and Wuest, W.M., ACS Infect. Dis., 2015, vol. 1, p. 288. https://doi.org/10.1021/acsinfecdis.5b00047
Hegstad, K., Langsrud, S., Lunestad, B.T., Scheie, A.A., Sunde, M., and Yazdankhah, S.P., Microb. Drug Resist., 2010, vol. 16, p. 91. https://doi.org/10.1089/mdr.2009.0120
Sapozhnikov, S.V., Shtyrlin, N.V., Kayumov, A.R., Zamaldinova, A.E., Iksanova, A.G., Nikitina, Е.V., Krylova, Е.S., Grishaev, D.Yu., Balakin, K.V., and Shtyrlin, Yu.G., Med. Chem. Res., 2017, vol. 26, p. 3188. https://doi.org/10.1007/s00044-017-2012-9
Vereshchagin, A.N., Frolov, N.A., Egorova, K.S., Seitkalieva, M.M., and Ananikov, V.P., Int. J. Mol. Sci., 2021, vol. 22, p. 6793. https://doi.org/10.3390/ijms22136793
Knauf, G.A., Cunningham, A.L., Kazi, M.I., Riddington, I.M., Crofts, A.A., Cattoir, V., Trent, M.S., and Davies, B.W., mBio, 2018, vol. 9, p. e02394-17. https://doi.org/10.1128/mBio.02394-17
Chauret, C.P., Encycl. Food Microbiol., 2014, vol. 3, p. 360. https://doi.org/10.1016/B978-0-12-384730-0.00407-9
Kwasniewska, D., Chen, Y.-L., and Wieczorek, D., Pathogens, 2020, vol. 9, p. 459. https://doi.org/10.3390/pathogens9060459
Xu, Q., Hu, X., and Wang, Y., Molecular Biotechnol., 2021, vol. 63, p. 1103. https://doi.org/10.1007/s12033-021-00371-2
Druvari, D., Koromilas, N.D., Bekiari, V., Bokias, G., and Kallitsis, J.K., Coatings, 2018, vol. 8, p. 8. https://doi.org/10.3390/coatings8010008
Jiao, Y., Niu, L., Ma, S., Li, J., Tay, F.R., and Chen, J., Progr. Polym. Sci., 2017, vol. 71, p. 53. https://doi.org/10.1016/j.progpolymsci.2017.03.001
Xue, Y., Xiao, H., and Zhang, Y., Int. J. Mol. Sci., 2015, vol. 16, p. 3626. https://doi.org/10.3390/ijms16023626
Hrubec, T.C., Seguin, R.P., Xu, L., Cortopassi, G.A., Datta, S., Hanlon, A.L., Lozano, A.J., McDonald, V.A., Healy, C.A., Anderson, T.C., Musse, N.A., and Williams, R.T., Toxicology Rep., 2021, vol. 8, p. 646. https://doi.org/10.1016/j.toxrep.2021.03.006
Di Nica, V., Gallet, J., Villa, S., and Mezzanotte, V., Ecotoxicol. Environ. Safety, 2017, vol. 142, p. 567. https://doi.org/10.1016/j.ecoenv.2017.04.028
Ogata, M., Tutumimoto Sato, K., Kunikane, T., Oka, K., Seki, M., Urano, Sh., Hiramatsu, K., and Endo, T., Biol. Pharm. Bull., 2005, vol. 28, p. 1120. https://doi.org/10.1248/bpb.28.1773
Selassie, C.D., Verma, R.P., Kapur, S., Shusterman, A.J., and Hansch, C., J. Chem. Soc. Perkin Trans. 2, 2002, p. 1112. https://doi.org/10.1039/b201478e
Starodubtseva, R.R., Gibadullina, E.M., Pazilova, N.B., Sapunova, A.S., Voloshina, A.D., Sudakov, I.A., Vyshtakalyuk, A.B., Pudovik, M.A., Burilov, A.R., and Bukharov, S.V., Med. Chem. Commun., 2018, vol. 9, p. 2106. https://doi.org/10.1039/c8md00481a
Bogdanov, A.V., Zaripova, I.F., Voloshina, A.D., Strobykina, A.S., Kulik, N.V., Bukharov, S.V., and Mironov, V.F., Russ. J. Gen. Chem., 2018, vol. 88, p. 57. https://doi.org/10.1134/S1070363218010097
Bogdanov, A.V., Iskhakova, K.R., Voloshina, A.D., Sapunova, A.S., Kulik, N.V., Terekhova, N.V., Arsenyev, M.V., Ziyatdinova, G.K., and Bukharov, S.V., Chem. Biodivers., 2020, vol. 17. e2000147. https://doi.org/10.1002/cbdv.202000147
Bogdanov, A.V., Zaripova, I.F., Voloshina, A.D., Strobykina, A.S., Kulik, N.V., Bukharov, S.V., Voronina, Ju.K., Khamatgalimov, A.R., and Mironov, V.F., Monatsh. Chem., 2018, vol. 149, p. 111. https://doi.org/10.1007/s00706-017-2049-y
Bogdanov, A.V., Zaripova, I.F., Voloshina, A.D., Sapunova, A.S., Kulik, N.V., Bukharov, S.V., Voronina, Ju.K., Vandyukov, A.E., and Mironov, V.F., ChemistrySelect, 2019, vol. 4, p. 6162. https://doi.org/10.1002/slct.201901708
Bogdanov, A.V., Kadomtseva, M.E., Bukharov, S.V., Voloshina, A.D., and Mironov, V.F., Russ. J. Org. Chem., 2020, vol. 56, p. 555. https://doi.org/10.1134/S107042802003032X
Bogdanov, A.V., Voloshina, A.D., Khamatgalimov, A.R., Terekhova, N.V., and Mironov, V.F., Doklady Chem., 2020, vol. 494, no. 1, p. 136. https://doi.org/10.1134/S0012500820090013
Syakaev, V.V., Podyachev, S.N., Buzykin, B.I., Latypov, Sh.K., Habicher, V.D., and Konovalov, A.I., J. Mol. Struct., 2006, vol. 788, p. 55. https://doi.org/10.1016/j.molstruc.2005.11.018
Arsenyev, M.V., Baranov, E.V., Fedorov, A.Yu., Chesnokov, S.A., and Abakumov, G.A., Mendeleev Commun., 2015, vol. 25, p. 312. https://doi.org/10.1016/j.mencom.2015.07.029
Arsenyev, M.V., Khamaletdinova, N.M., Baranov, E.V., Chesnokov, S.A., and Cherkasov, V.K., Russ. Chem. Bull., 2016, vol. 65, p. 1805. https://doi.org/10.1007/s11172-016-1514-9
Levison, L.J., Miller-Cushon, E.K., Tucker, A.L., Bergeron, R., Leslie, K.E., Barkema, H.W., and De Vries, T.J., J. Dairy Sci., 2016, vol. 99, p. 1341. https://doi.org/10.3168/jds.2015-9809
Vakkamаki, J., Taponen, S., Heikkila, A.-M., and Pyorala, S., Acta Vet. Scand., 2017, vol. 59, p. 33. https://doi.org/10.1186/s13028-017-0301-4
Verbeke, J., Piepers, S., Supre, K., and De Vliegher, S., J. Dairy Sci., 2014, vol. 97, p. 6926. https://doi.org/10.3168/jds.2014-8173
Pyorala, S. and Taponen, S., Vet. Microbiol., 2009, vol. 134, p. 3. https://doi.org/10.1016/j.vetmic.2008.09.015
Simojoki, H., Salomaki, T., Taponen, S., Iivanainen, A., and Pyorala, S., Vet. Res., 2011, vol. 42, p. 49. https://doi.org/10.1186/1297-9716-42-49
Felipe, V., Breser, M. L., Bohl, L.P., Rodrigues da Silva, E., Morgante, C.A., Correa, S.G., and Porporatto, C., Int. J. Biol. Macromol., 2019, vol. 126, p. 60. https://doi.org/10.1016/j.ijbiomac.2018.12.159
Bogdanov, A., Tsivileva, O., Voloshina, A., Lyubina, A., Amerhanova, S., Burtceva, E., Bukharov, S., Samorodov, A., and Pavlov, V., ADMET & DMPK, 2022, vol. 10, p. 163. https://doi.org/10.5599/admet.1179
Wang, T., Gao, C., Cheng, Y., Li, Z., Chen, J., Guo, L., and Xu, J., Plants, 2020, vol. 9, p. 769. https://doi.org/10.3390/plants9060769
Mastanjevic, K., Krstanovic, V., Mastanjevic, K., and Sarkanj, B., Fermentation, 2018, vol. 4, p. 3. https://doi.org/10.3390/fermentation4010003
Han, J.-H., Park, G.-C., and Kim, K.S., Mycobiology, 2017, vol. 45, p. 370. https://doi.org/10.5941/MYCO.2017.45.4.370
Lim, B., Cheng, Y., Kato, T., Pham, A.T., Le Du, E., Mishra, A.K., Grinhagena, E., Moreau, D., Sakai, N., Waser, J., and Matile, S., Helv. Chim. Acta, 2021, vol. 104. e2100085. https://doi.org/10.1002/hlca.202100085
Abd-El-Khair, H., Abdel-Gaied, T.G., Mikhail, M.S., Abdel-Alim, A.I., and El-Nasr, H.I.S., Bull. Nat. Res. Centre, 2021, vol. 45, p. 37. https://doi.org/10.1186/s42269-021-00491-4
Huang, X., Ren, J., Li, P., Feng, S., Dong, P., and Ren, M., J. Sci. Food Agric., 2021, vol. 101, p. 1744. https://doi.org/10.1002/jsfa.10829
Ragasova, L., Penazova, E., Gazdik, F., Pecenka, J., Cechova, J., Pokluda, R., Baranek, M., Grzebelus, D., and Eichmeier, A., Agronomy, 2020, vol. 10, p. 443. https://doi.org/10.3390/agronomy10030443
Davidson, C.M. and Cronin, F., Appl. Microbiol., 1973, vol. 26, p. 439. https://doi.org/10.1128/am.26.3.439-440.1973
McCoy, R.H. and Pilcher, K.S., J. Fish. Board Canada, 1974, vol. 31, p. 1553. https://doi.org/10.1139/f74-193
Essenberg, M., Doherty, M.D.A., Hamilton, B.K., Henning, V.T., Cover, E.C., McFaul, S.J., and Johnson, W.M., Phytopathology, 1982, vol. 72, p. 1349. https://doi.org/10.1094/Phyto-72-1349
Ming, D., Ye, H., Schaad, N.W., and Roth, D.A., Phytopathology, 1991, vol. 81, p. 1358. https://doi.org/10.1094/Phyto-81-1358
ACKNOWLEDGMENTS
The authors are grateful to the Spectro-Analytical Center of the Kazan Scientific Center of the Russian Academy of Sciences for the technical support of the research.
Funding
The work was supported by the Strategic Academic Leadership Program of the Kazan (Volga Region) Federal University (Prioritet-2030). The study of antiphytopathogenic activity was carried out within the framework of the Program of Fundamental Scientific Research of the Russian Academy of Sciences (subject No. 121031100266-3, O.M. Tsivileva).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
To Memory of V.I. Galkin
Rights and permissions
About this article
Cite this article
Bogdanov, A.V., Bukharov, S.V., Garifullina, R.A. et al. Synthesis and Antimicrobial Activity Evaluation of Ammonium Acylhydrazones Based on 4,6-Di-tert-butyl-2,3-dihydroxybenzaldehyde. Russ J Gen Chem 92, 1875–1886 (2022). https://doi.org/10.1134/S1070363222100012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222100012