Abstract
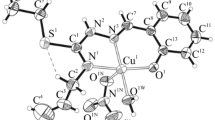
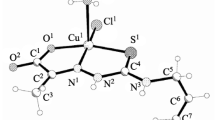
The reaction of N-(prop-2-en-1-yl)hydrazinecarbothioamide with substituted 2-hydroxybenzaldehydes afforded the corresponding Schiff bases which were used as ligands to obtain copper and cobalt coordination compounds Cu(NL1–6)X · n H2O (X = Cl−, \(\rm{NO}_3^-\); n = 0–3), Co(HL2)2NO3, and Co(NL6)2Cl. The structure of the isolated complexes was determined by NMR spectroscopy and X-ray analysis. The complexes were tested for antimicrobial and antifungal activity against S. aureus, E. coli, and yeast-like fungi. Inhibitory effect of the initial thioamides and their complexes against human myeloid leukemia HL-60 cancer cell line was also studied.
Similar content being viewed by others
References
Bal-Demirci, T., Polyhedron, 2008, vol. 27, p. 440. doi https://doi.org/10.1016/j.poly.2007.10.001
Orysyk, S.I., Bon, V.V., Zholob, O.O., Pekhnyo, V.I., Orysyk, V.V., Zborovskii, Yu.L., and Vovk, M.V., Polyhedron, 2013, vol. 51, p. 211. doi https://doi.org/10.1016/j.poly.2012.12.021
Bon, V.V., Orysyk, S.I., and Pekhnyo, V.I., Russ. J. Coord. Chem., 2011, vol. 37, no. 2, p. 149. doi https://doi.org/10.1134/s1070328411010027
Bon, V.V., Acta Crystallogr., Sect. C, 2010, vol. 66, p. 300. doi https://doi.org/10.1107/S0108270110035754
Orysyk, S.I., Bon, V.V., Obolentseva, O.O., Zborovskii, Yu.L., Orysyk, V.V., Pekhnyo, V.I., Staninets, V.I., and Vovk, M.V., Inorg. Chim. Acta, 2012, vol. 382, p. 127. doi https://doi.org/10.1016/j.ica.2011.10.027
Orysyk, S.I., Repich, G.G., Bon, V.V., Dyakonenko, V.V., Orysyk, V.V., Zborovskii, Yu.I., Shishkin, O.V., Pekhnyo, V.I., and Vovk, M.V, Inorg. Chim. Acta, 2014, vol. 423, p. 496. doi https://doi.org/10.1016/j.ica.2014.08.056
Kalinowski, D.S., Quach, P., and Richardson, D.R., Future Med. Chem., 2009, vol. 1, no. 6, p. 1143. doi https://doi.org/10.4155/FMC.09.80
Lovejoy, D.B. and Richardson, D.R., Blood, 2002, vol. 100, p. 666. doi https://doi.org/10.1182/blood.V100.2.666
Ülküseven, B., Bal-Demirci, T., Akkurt, M., Yalçin, Ş.P., and Büyükgüngör, O., Polyhedron, 2008, vol. 27, no. 18, p. 3646. doi https://doi.org/10.1016/j.poly.2008.08.024
Scott, A.W. and McCall, M.A., J. Am. Chem. Soc., 1945, vol. 67, no. 10, p. 1767. doi https://doi.org/10.1021/ja01226a043
Bal-Demirci, T., Akkurt, M., Yalcm, S.P., and Buyukgungor, O., Transition Met. Chem., 2010, vol. 35, p. 95. doi https://doi.org/10.1007/s11243-009-9300-2
Duan, C.-Y., Tian, Y.-P., Zhao, C.-Y., You, X.-Z., and Mak, T.C.W., Polyhedron, 1997, vol. 16, p. 2857. doi https://doi.org/10.1016/S0277-5387(97)00013-2
Vrdoljak, V., Cindric, M., Milic, D., Dubravka, M.C., Predrag, N., and Kamenar, B., Polyhedron, 2005, vol. 24, p. 1717. doi https://doi.org/10.1016/j.poly.2005.05.002
Bourosh, P.N., Revenko, M.D., Gdaniec, M., Stratulat, E.F., and Simonov, Yu.A., J. Struct. Chem., 2009, vol. 50, no. 3, p. 510. doi https://doi.org/10.1007/s10947-009-0078-z
Chumakov, Yu.M., Biyushkin, V.N., and Bodyu, V.G., J. Struct. Chem., 1985, vol. 26, no. 6, p. 929. doi https://doi.org/10.1007/BF00748365
Spek, A.L., J. Appl. Crystallogr., 2003, vol. 36, p. 7. doi https://doi.org/10.1107/S0021889802022112
Malone, J.F., Murray, C.M., Charlton, M.H., Docherty, R., and Lavery, A.J., J. Chem. Soc., Faraday Trans., 1997, vol. 93, p. 3429. doi https://doi.org/10.1039/A700669A
CrysAlisPro, Version 1.171.33.52 (release 06-11-2009 CrysAlis171.NET). Oxford Diffraction.
Sheldrick, G.M., Acta Crystallogr., Sect. A, 2008, vol. 64, p. 112. doi https://doi.org/10.1107/S0108767307043930
Allen, F.H., Acta Crystallogr., Sect. B, 2002, vol. 58, p. 380. doi https://doi.org/10.1107/S0108768102003890
Addison, A.W., Rao, T.N., Reedijk, J., and Verschoor, G.C., J. Chem. Soc., Dalton Trans., 1984, no. 7, p. 1349. doi https://doi.org/10.1039/DT9840001349
Gulea, A., Poirier, D., Roy, J., Stavila, V., Bulimestru, I., Tapcov, V., Birca, M., and Popovschi, L., J. Enzyme Inhib. Med. Chem., 2008, vol. 23, no. 6, p. 806. doi https://doi.org/10.1080/147563607017443002
Acknowledgments
The authors thank Prof. D. Poirier (Laval University, Quebec, Canada) and O.S. Garbuz for their help in performing biological testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © The Authors(s), 2019, published in Zhurnal Obshchei Khimii, 2019, Vol. 89, No. 5, pp. 766–778.
Rights and permissions
About this article
Cite this article
Gulea, A.P., Graur, V.O., Chumakov, Y.M. et al. Synthesis, Structure, and Biological Activity of Copper and Cobalt Coordination Compounds with Substituted 2-(2-Hydroxybenzylidene)-N-(prop-2-en-1-yl)hydrazinecarbothioamides. Russ J Gen Chem 89, 953–964 (2019). https://doi.org/10.1134/S1070363219050153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219050153