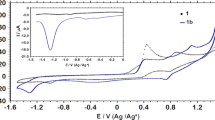
Abstract
Hydrogen transfer hydrogenation of acetophenone and methyl benzoylformate with 2-propanol was studied on colloidal systems obtained by reduction of rhodium complexes in the presence of optically active compounds: chiral diamines, quaternary salt (4S,5S)-(–)-N 1,N 4-dibenzylene-2,3-dihydroxy-N 1,N 1,N 4,N 4-tetramethylbutane-1,4-diammonium dichloride and (8S,9R)-(–)-cinchonidine. The increase in the molar ratio modifier/Rh leads to the increase in the enantioneric excess (ee) of the reaction products. The largest ee [43.8% of (R)-1-phenylethanol and 58.2% of methyl ester of (R)-mandelic acid] were achieved for the ratios (8S,9R)-(–)-cinchonadine: Rh = 9: 1 and 3: 1, respectively. The catalyst was characterized by the high-resolution transmission electron microscopy, X-ray diffraction analysis, and thermal analysis.
Similar content being viewed by others
References
Ott, L.S. and Finke, R.G., Coord. Chem. Rev., 2007, vol. 251, nos. 9–10, p. 1075. DOI: 10.1016/jccr.2006.08.016.
Widegren, J.A. and Finke, R.G., J. Mol. Catal. (A), 2003, vol. 191, no. 2, p. 187. DOI: 10.1016/S1381-1169 (02)00125-5.
Yan, N., Xiao, C., and Kou, Y., Coord. Chem. Rev., 2010, vol. 254, nos. 9–10, p. 1179. DOI: 10.1016/jccr.2010.02.015.
Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control, Corain, B., Schmid, G., and Toshima, N., Eds., Amsterdam Elsevier, 2008.
Mahmoud, M.A., Narayanan, R., and El-Sayed, M.A., Acc. Chem. Res., 2013, vol. 46, no. 8, p. 1795. DOI: 10.1021/ar3002359.
Yan, N., Yuan, Y., and Dyson, P., Dalton Trans., 2013, vol. 42, no. 37, p. 13294. DOI: 10.1039/C3DT51180D.
Chaudhuri, R.G. and Paria, S., Chem. Rev., 2012, vol. 112, no. 4. 2373. DOI: 10.1021/cr100449n.
Cookson, J., Platinum Metals Rev., 2012, vol. 56, no. 2, p. 83. DOI: 10.1595/147106712x632415.
Pushkarev, V.V., Zhu, Z., An, K., Hervier, A., and Somorjai, G.A., Top. Catal., 2012, vol. 55, nos. 19–20, p. 1257. DOI: 10.1007/s11244-012-9915-y.
Bucher, S., Hormes, J., Modrow, H., Brinkmann, R., Waldöfner, N., Bönnemann, H., Beuermann, L., Krischok, S., Maus-Friedrichs, W., and Kempter, V., Surface Sci., 2002, vol. 497, nos. 1–3, p. 321. DOI: 10.1016/S0039-6028(01)01663-6.
Zeng, J., Tao, J., Li, W., Grant, J., Wang, P., Zhu, Y., and Xia, Y., Chem. Asian J., 2011, vol. 6, no. 2, p. 376. DOI: 10.1002/asia.201000728.
Roucoux, A., Schulz, J., and Patin, H., Adv. Synth. Catal., 2003, vol. 345, nos. 1–2, p. 222. DOI: 10.1002/adsc.200390016.
Park, K.H., Jang, K., and Kim, H.J., Son, S.U., Angew. Chem. Int. Ed., 2007, vol. 46, no. 7. 1152. DOI: 10.1002/anie.200603961.
Zhou, K.B. and Li, Y.D., Angew. Chem. Int. Ed., 2012, vol. 51, no. 3, p. 602. DOI: 10.1002/anie.201102619.
Weddle, K.S., Aiken, J.D., and Finke, R.G., J. Am. Chem. Soc., 1998, vol. 120, no. 23, p. 5653. DOI: 10.1021/ja973045h.
Torsi, L., Farinola, G.M., Marinelli, F., Tanese, M.C., Omar, O.H., Valli, L., Babudri, F., Palmisano, F., Zambonin, P.G., and Naso, F., Nat. Mater., 2008, vol. 7, no. 5, p. 412. DOI: 10.1038/nmat2167.
Saha, K., Agasti, S.S., Kim Ch., Li, X., and Rotello, V.M., Chem. Rev., 2012, vol. 112, no. 5, p. 2739. DOI: 10.1021/cr2001178.
Somorjai, G.A. and Li, Y., Top. Catal., 2010, vol. 53, nos. 13–14, p. 832. DOI: 10.1007/s11244-010-9511-y.
Yasukawa, T., Miyamura, H., and Kobayashi, S., J. Am. Chem. Soc., 2012, vol. 134, no. 41, p. 16963. DOI: 10.1021/ja307913e.
Stowell, C.A. and Korgel, B.A., Nano Lett., 2005, vol. 5, no. 7, p. 1203. DOI: 10.1021/nl050648f.
Baiker, A., Catal. Today., 2005, vol. 100, nos. 1–2, p. 159. DOI: 10.1016/jcattod.2004.12.001.
Nanostructured Catalysts, Scott, S., Ed., New York: Kluwer Academic. Plenum Publishers, 2003, p. 179.
Wells, P.B. and Wilkinson, A.G., Top. Catal., 1998, vol. 5, nos. 1–4, p. 39. DOI: 10.1023/A:1019197802585.
Barbaro, P., Santo, V.D., and Liguori, F., Dalton Trans., 2010, vol. 39, no. 36, p. 8391. DOI: 10.1039/C002051F.
Baiker, A., J. Mol. Catal. (A), 2000, vol. 163, no. 1, p. 205. DOI: 10.1016/S1381-1169(00)00387-3.
Arx, M., Mallat, T., and Baiker, A., Top. Catal., 2002, vol. 19, no. 1, p. 75. DOI: 10.1023/A:1013885300523.
Blaser, H.-U. and Studer, M., Acc. Chem. Res., 2007, vol. 40, no. 12, p. 1348. DOI: 10.1021/ar700088f.
Blaser, H.-U., Jalett, H.P., Lottenbach, W., and Studer, M., J. Am. Chem. Soc., 2000, vol. 122, no. 51, p. 12675. DOI: 10.1021/ja003259q.
Burgi, T. and Baiker, A., Acc. Chem. Res., 2004, vol. 37, no. 11, p. 909. DOI: 10.1021/ar040072l.
Margitfalvi, J.L., Tálas, E., and Tfirst, E., Top. Catal., 2006, vol. 39, nos. 1–2, p. 77. DOI: 10.1007/s11244- 006-0040-7.
Murzin, D.Yu. and Toukoniitty, E., React. Kinet. Catal. Lett., 2007, vol. 90, no. 1, p. 19. DOI: 10.1007/s11144- 007-5004-9.
Kraynov, A. and Richards, R., Phys. Chem. Chem. Phys., 2007, vol. 9, no. 7, p. 884. DOI: 10.1039/B615470K.
Studer, M., Blaser, H.-U., and Okafor, V., Chem. Commun., 1998, no. 9, p. 1053. DOI: 10.1039/a801390j.
Gómez, M., Philippot, K., Colliére, V., Lecante, P., Mullera, G., and Chaudret, B., New J. Chem., 2003, vol. 27, no. 1, p. 114. DOI: 10.1039/B206660M.
Sun, Y., Guo, Y., Lu, Q., Meng, X., Xiaohua, W., Guo, Y., Wang, Y., Liu, X., and Zhang, Z., Catal. Lett., 2005, vol. 100, nos. 3–4, p. 213. DOI: 10.1007/s10562-004- 3458-1.
Xue, P. and Wu, T., Front. Chem. Eng. China, 2007, vol. 1, no. 3, p. 251. DOI: 10.1007/s11705-007-0045-1.
Roucoux, A., Schulz, J., and Henri, P., Chem. Rev., 2002, vol. 102, no. 10, p. 3757. DOI: 10.1021/cr010350j.
Jansat, S., Picurelli, D., Pelzer, K., Philippot, K., Gómez, M., Muller, G., Lecante, P., and Chaudret, B., New J. Chem., 2006, vol. 30, no. 1, p. 115. DOI: 10.1039/B509378C.
Nindakova, L.O., Chipanina, N.N., Turchaninov, V.K., Ustinov, M.V., and Shainyan, B.A., Russ. Chem. Bull., 2005, vol. 54, no. 10, p. 2343. DOI: 10.1007/s11172- 006-0120-7.
Axet, M.R., Castillon, S., Claver, C., Philippot, K., Lecante, P., and Chaudret, B., Eur. J. Inorg. Chem., 2008, no. 22, p. 3460. DOI: 10.1002/ejic.200800421.
Ewers, T.D., Sra, A.K., Norris, B.C., Cable, R.E., Cheng, C.-H., Shantz, D.F., and Schaak, R.E., Chem. Mater., 2005, vol. 17, no. 3, p. 514. DOI: 10.1021/cm0483792.
Glavee, G.N., Klabunde, K.J., Sorensen, C.M., and Hadjipanayis, G.C., Langmuir., 1993, vol. 9, no. 1, p. 162. DOI: 10.1021/la00025a034.
Boström, M., Williams, D.R.M., and Ninham, B.W., Phys. Rev. Lett., 2001, vol. 87, no. 16, p. 168103 DOI: 10.1103/PhysRevLett.87.168103.
Bucher, S., Hormes, J., Modrow, H., Brinkman, R., Waldöfner, N., Bönneman, H., Beuermann, L., Krischok, S., Maus-Friedrichs, W., and Kempter, V., Surf. Sci., 2002, vol. 497, nos. 1–3, p. 321. DOI: 10.1016/S0039-6028(01)01663-6.
Bonnemann, H., Braun, G., Brijoux, W., Brinkmann, R., Schulze, T.A., Seevogel, K., and Siepen, K., J. Organomet. Chem., 1996, vol. 520, nos. 1–2, p. 143. DOI: 10.1016/0022-328X(96)06273-0.
Metal Nanoparticles: Synthesis, Characterization and Applications, Feldheim, D.L. and Foss, C.A. Jr., Eds., New York: Marcel Dekker, 2002, p. 17.
Szöllösi, G., Cserényi, S., Balázsik, K., Fülöp, F., and Bartók, M., J. Mol. Catal. (A), 2009, vol. 305, nos. 1–2, p. 155. DOI: 10.1016/jmolcata.2009.01.030.
Narayanan, R. and El-Sayed, M., J. Phys. Chem. (B), 2004, vol. 108, no. 25, p. 8572. DOI: 10.1021/jp037169u.
Gniewek, A. and Trzeciak, A.M., Top. Catal., 2013, vol. 56, no. 13, p. 1239. DOI: 10.1007/s11244-013-0090-6.
Semagina, N., Renken, A., and Kiwi-Minsker, L., J. Phys. Chem. (C), 2007, vol. 111, no. 37, p. 13933. DOI: 10.1021/jp073944k.
Murata, K., Ikariya, T., and Noyori, R., J. Org. Chem., 1999, vol. 64, no. 7, p. 2186. DOI: 10.1021/jo990213a.
Touchard, F., Bernard, M., Fache, F., Delbecq, F., Guiral, V., Sautet, P., and Lemaire, M., J. Organomet. Chem., 1998, vol. 567, nos. 1–2, p. 133. DOI: 10.1016/S0022-328X(98)00675-5.
Seebach, D., Kalinowski, H.O., Bastini, B., Crass, G., Daum, H., Doerr, H., DuPreez, N.P., and Ehrig, V., Langer, W., Helv. Chim. Acta., 1977, vol. 60, no. 2, p. 301. DOI: 10.1002/hlca., 19770600202.
Nindakova, L.O., Shainyan, B.A., and Belonogova, L.N., Russ. J. Org. Chem., 2003, vol. 39, no. 10, p. 1484. DOI: 10.1023/B:RUJO.0000010566.99408.65.
Chambers, W.J., Brasen, W.R., and Hause, C.R., J. Am. Chem. Soc., 1957, vol. 79, no. 4, p. 879. DOI: 10.1021/ja01561a025.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.O. Nindakova, N.М. Badyrova, V.V. Smirnov, V.О. Strakhov, S.S. Kolesnikov, 2016, published in Zhurnal Obshchei Khimii, 2016, Vol. 86, No. 6, pp. 909–918.
Rights and permissions
About this article
Cite this article
Nindakova, L.O., Badyrova, N.M., Smirnov, V.V. et al. Enantioselective hydrogen transfer hydrogenation on rhodium colloid systems with optically active stabilizers. Russ J Gen Chem 86, 1240–1249 (2016). https://doi.org/10.1134/S1070363216060049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363216060049