Abstract
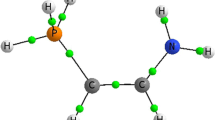
A theoretical analysis of the geometry of the isolated homoconjugated ion formed by interaction of the pyridine molecule with the pyridinium cation was performed. With no o-substituents and no external electric field applied, the hydrogen bond is strongly asymmetric and is characterized by a low barrier to proton transfer between the two chemically equivalent structures. According to the calculations, the most probable averaged distance between the nitrogen atoms forming the hydrogen bond in such homoconjugated cations is about 2.7 ± 0.1 Å.
Similar content being viewed by others
References
Andreeva, D.V., Ip, B., Gurinov, A.A., Tolstoy, P.M., Denisov, G.S., Shenderovich, I.G., and Limbach, H.H., J. Phys. Chem. (A), 2006, vol. 110, no. 37, p. 10872.
Golubev, N.S., Shenderovich, I.G., Smirnov, S.N., Denisov, G.S., and Limbach, H.H., Chem. Eur. J., 1999, vol. 5, no. 2, p. 492.
Shenderovich, I.G., Burtsev, A.P., Denisov, G.S., Golubev, N.S., and Limbach, H.H., Magn. Reson. Chem., 2001, vol. 39, p. S91.
Tolstoy, P.M., Smirnov, S.N., Shenderovich, I.G., Golubev, N.S., Denisov, G.S., and Limbach, H.H., J. Mol. Struct., 2004, vol. 700, nos. 1–3, p. 19.
Limbach, H.H., Pietrzak, M., Sharif, S., Tolstoy, P.M., Shenderovich, I.G., Smirnov, S.N., Golubev, N.S., and Denisov, G.S., Chem. Eur. J., 2004, vol. 10, no. 20, p. 5195.
Schah-Mohammedi, P., Shenderovich, I.G., Detering, C., Limbach, H.H., Tolstoy, P.M., Smirnov, S.N., Denisov, G.S., and Golubev, N.S., J. Am. Chem. Soc., 2000, vol. 122, no. 51, p. 12878.
Guzei, I.A., Roberts, J., and Saulys, D.A., Acta Crystallogr., Sect. C, 2002, vol. 58, no. 3, p. M141.
Odinokov, S.E., Mashkovsky, A.A., and Nabiullin, A.A., Spectrochim. Acta (A), 1983, vol. 39, no. 12, p. 1065.
Villarreal-Salinas, B.E. and Schlemper, E.O., J. Cryst. Mol. Struct., 1978, vol. 8, no. 5, p. 217.
Brzezinski, B. and Zundel, G., J. Chem. Soc., Faraday Trans. 2, 1976, vol. 72, no. 12, p. 2127.
Quick, A., Williams, D.J., Borah, B., and Wood, J.L., J. Chem. Soc., Chem. Commun., 1974, no. 21, p. 891.
Del Bene, J.E., Bartlett, R.J., and Elguero, J., Magn. Reson. Chem., 2002, vol. 40, no. 12, p. 767.
Shenderovich, I.G., Zh. Obshch. Khim., 2006, vol. 76, no. 4, p. 529.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A., Jr., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Rega, N., Salvador, P., Dannenberg, J.J., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S., and Pople, J.A., GAUSSIAN 98, Rev. A.11.3, Pittsburgh PA: Gaussian, 2002.
Larson, J.W. and McMahon, T.B., Inorg. Chem., 1984, vol. 23, no. 14, p. 2029.
Caldwell, G. and Kebarle, P., Can. J. Chem., 1985, vol. 63, no. 7, p. 1399.
Lorente, P., Shenderovich, I.G., Golubev, N.S., Denisov, G.S., Buntkowsky, G., and Limbach, H.H., Magn. Reson. Chem., 2001, vol. 39, special issue, p. S18.
Shenderovich, I.G., Buntkowsky, G., Schreiber, A., Gedat, E., Sharif, S., Albrecht, J., Golubev, N.S., Findenegg, G.H., and Limbach, H.H., J. Phys. Chem. (B), 2003, vol. 107, no. 43, p. 11924.
Golubev, N.S., Melikova, S.M., Shchepkin, D.N., Shenderovich, I.G., Tolstoy, P.M., and Denisov, G.S., Z. Phys. Chem., 2003, vol. 217, no. 12, p. 1549.
Author information
Authors and Affiliations
Additional information
Original Russian Text © I.G. Shenderovich, 2007, published in Zhurnal Obshchei Khimii, 2007, Vol. 77, No. 4, pp. 663–668.
Rights and permissions
About this article
Cite this article
Shenderovich, I.G. Qualitative analysis of the geometry of the hydrogen bond in the homoconjugated pyridine ion. Russ J Gen Chem 77, 620–624 (2007). https://doi.org/10.1134/S1070363207040202
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070363207040202