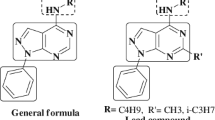
Abstract
Reaction of 4,6-dimethyl-3-cyanopyridine-2(1H)-thione (IIIa) or 4,5,6-trisubstituted-3-cyanopyridine-2(1H)-thiones (IIIb–d) with 2-chloromethylquinazoline-4(3H)-one (IVa) furnished the corresponding 3-amino-2-(4-oxo-3,4-dihydroquinazolin-2-yl)thieno[2,3-b]pyridines (VIa–d). Reaction of aminothieno-pyridines (VIa, b, d) were reacted with triethyl orthoformate, acetic anhydride or nitrous acid to furnish pyridothienopyrimidoquinazolines (VIIIa, b, d), (IXa, b, d) or pyridothienotriazinoquinazolines (Xa, b, d). The new compound, 3-cyano-5-acetyl-6-methyl-4-styrylpyridine-2(1H)-thione (IIIe) was synthesized and reacted with 2-chloromethyl-1H-benzimadazole to give 5-acetyl-3-amino-2-(1H-benzimidazol-2-yl)-6-methyl-4-styryl-thieno[2,3-b]pyridine (XII) which was used as a key intermediate for synthesizing pyridothienopyrimidobenzimidazoles (XIII, XIV). All newly synthesized compounds were characterized on the basis of their elemental and spectral analyses. Also, most of the synthesized compounds were screened in vitro for their antifungal activity.
Similar content being viewed by others
REFERNCES
Henry, G. D., Tetrahedron, 2004, vol. 60, pp. 6043–6061. https://doi.org/10.1016/j.tet.2004.04.043
Altaf, A. A., Shahzad, A., Gul, Z., Rasool, N., Badshah, A., Khan, E., and Lal, B., J. Drug Design Med. Chem., 2015, vol. 1, pp.1–11. https://doi.org/10.11648/j.jddmc.20150101.11
Bakhite, E.A., Phosphorus, Sulfur Silicon Relat. Elem., 2003, vol. 178, pp. 929–992. https://doi.org/10.1080/10426500307855
Litvinov, V.P., Dotsenko, V.V., and Krivokolysko, S.G., Russ. Chem. Bull. Int. Ed., 2005, vol. 54, pp. 864–904. https://doi.org/10.1007/s11172-005-0333-1
Schnute, M.E., Anderson, D.J., Brideau, R.J., Ciske, E.L., Collier, S.A., Cudahy, M.M., Eggen, M., Genin, M.J., Hopkins, T.A., Judge, T.M., Kim, E.J., Knechtel, M.L., Nair, S.K., Nieman, J.A., Oien, N.L., Scott, A., Tanis, S.P., Vaillancount, V.A., Wathen, M.W., and Wieber, J.L., 2007, Bioorg. Med. Chem. Lett., 2007, vol. 17, pp. 3349–3353. https://doi.org/10.1016/j.bmcl.2007.03.102
Attaby, F.A., Elghandour, A.H.H., Ali, M.A., and Ibrahem, Y.M., Phosphorus, Sulfur Silicon Relat. Elem., 2007, vol. 182, 695–709. https://doi.org/10.1080/10426500601087277
Bahekar, R.H., Jain, M.R., Jadav, P.A., Prajapati, V.M., Patel, D.N., Gupta, A.A., Sharma, A., Tom, R., Bandyopadhya, D., Modi, H., and Patel, P.R., Bioorg. Med. Chem. 2007, vol. 15, pp. 6782–6795. https://doi.org/10.1016/j.bmc.2007.08.00
Abdel-Rahman, A.E., Bakhite, E.A., and Al-Taifi, E.A., Pharmazie, 2003, vol. 58, pp. 372–377.
Hussin, A.M., Abu-Shanab, F.A., and Ishak, E.A., Phosphorus, Sulfur Silicon Relat. Elem., 2000, vol. 159, pp. 55–68. https://doi.org/10.1080/10426500008043650
Sable, P. and Ganguly, S., Indian J. Heterocycl. Chem., 2014, vol. 23, pp. 245–248.
Mohi El-Deen, E.M., Abd El-Meguid, E.A., Hasabelnaby, S., Karam, E.A., and Nossier, E.S., Molecules, 2019, vol. 24, pp. 3650–3669. https://doi.org/10.3390/molecules24203650
Madhusudana, K., Shireesha, B., Naidu, V.G.M., Ramakrishna, S., Narsaiah, B., Rao, A.R., and Diwan, P.V., Eur. J. Pharmacol., 2012, vol. 678, pp. 48–54. https://doi.org/10.1016/j.ejphar.2011.12.019
Hayakawa, I., Shioya, R., Agatsuma, T., Furukawa, H., and Sugano, Y., Bioorg. Med. Chem. Lett., 2004, vol. 14, pp. 3411–3414. https://doi.org/10.1016/j.bmcl.2004.04.079
Abouzid, K.A.M., Al-Ansary, G.H., El-Naggar, A.M., Eur. J. Med. Chem., 2017, vol. 134, pp. 357–365. https://doi.org/10.1016/j.ejmech.2017.04.024
Zeng, X.X., Zheng, R.-L., Zhou, T., He, H.-Y., Liu, J.-Y., Zheng, Y., Tong, A.-P., Xiang, M.-L., Song, X.-R., Yang, S.-Y., Wei, L.-T. Yu, Y.-Q., Zhao, Y.-L. and Yang, L. Bioorg. Med. Chem. Lett., 2010, vol. 20, pp. 6282–6285. https://doi.org/10.1016/j.bmcl.2010.08.088
Bernardino, A.M.R., Pinheiro, L.C., Rodrigues, C.R., Loureiro, N.L., Castro, H.C., Lanfredi-Rangel, A., Sabtini-Lopes, J., Borges, J.C., Carvalho, J.M., Romeiro, G.A., Ferreira, F.V., Fruguphetti, I.C.P.P., and Vannier Santos, M.A., Bioorg. Med. Chem., 2006, vol. 14, pp. 5765–5770. https://doi.org/10.1016/j.bmc.2006.03.013
Krauze, A., Germame, S., Eberlins, O., Sturms, I., Klusa, V., and Duburs, G., Eur. J. Med. Chem., 1999, vol. 34, 301–310.
Joshi, K.C. and Chand, P., J. Heterocycl. Chem., 1980, vol. 17, pp.1783–1784. https://doi.org/10.1002/jhet.5570170830
Furuya, S., Choho, N., Suzuki, N., and Imada, T., PCT Int. Appl. WO 00000493, 2000, Chem. Abstr., 2000, vol. 132, p. 64179.
Bridson, P.K., Davis, R.A., and Renner, L.S., J. Heterocycl. Chem., 1985, vol. 22, pp. 753–755. https://doi.org/10.1002/jhet.5570220328
Saito, T., Yasushi, M., Sakoshita, M., Toyda, K., and Shibazalti, T., Eur. Patent Appl., 535548, 1993, Chem. Abstr., 1993, vol. 119, p. 117112.
Gad-Elkareem, M.A.M., Abdel-Fattah, A.M. and Elneairy, M.A.A., Phosphorus, Sulfur Silicon Relat. Elem., 2006, vol. 181, pp. 891–911. https://doi.org/10.1080/10426500500272152
Doshi, H. Bhatt, M., and Thakkar, S., Am. J. Org. Chem., 2012, vol. 2, pp. 122–126. https://doi.org/10.5923/j.ajoc.20120205.03
Gautam, S., Mishra, D., Singh, R., and Pal, D.K., Int. J. Pharm., Chem. Biol. Sci., 2012, vol. 2, pp. 97–103.
Mohamed, M.S., Kamel, M.M., Kassem, E.M.M., Abotaleb, K.N.M., and Ahmed, M.F., Pharm. Drug Res., 2011, vol. 68, pp. 665–675.
Vashi, R.T., Shelat, C.D., and Pate, H., Int. J. Appl. Biol. Pharm. Techn., 2010, vol. 1, p. 883.
Vijaianand, P.R., Suresh, K. K., Sivakumar, R., Sam Solomon, W.D., and Jayaveera, K.N., Asian J. Chem., 2009, vol. 21, pp. 6656–6660.
Patel, H.U., Patel, R.S., and Patel, C.N., J. Appl. Pharm. Sci., 2013, vol. 3, pp. 171–174.
Mohamed, Y.A., El-galil, A., Amrb, C., Mohamed, S.F., Abdalla, M.M., Al- Omar, M.A., and Shfik, S.H., J. Chem. Sci., 2012, vol. 124, pp. 693–702. https://doi.org/10.1007/s12039-012-0242-4
Al-Omar, M.A., El-Azab, A.S., El-Obeid, H.A., and Abdel Hamide, S.G., J. Saudi Chem. Soc., 2006, vol. 10, pp. 1131–1139.
Sinha, N.K., Asnani, A.J., and Dravyakar, B.R., Asian J. Pharm. Clin. Res., 2013, vol. 6, pp. 200–204.
Mukherjee, D., Mukhopadhyay, A., Shridhara, K.B., Shridhara, A.M., and Rao, K.S., J. Pharm. Pharmaceut. Sci., 2014, vol. 6, pp. 567–571.
Sen, D., Banerjee, A., Ghosh, A.K., and Chatterjee, T.K., J. Adv. Pharm. Technol. Res., 2010, vol. 1, pp. 401–405. https://doi.org/10.4103/0110-5558.76439
El-Azab, A.S., Al-Omar, M.A., Abdel-Aziz, A.A.-M., Abdel-Aziz, N.I., El-Sayed, M.A.-A., Aleisa, A.M., Sayed-Ahmed, M.M., and Abdel-Hamide, S.G., Eur. J. Med. Chem., 2010, vol. 45, pp. 4188–4198. https://doi.org/10.1016/j.ejmech.2010.06.013
Srivastav, M.K. and Shantakumar, S.M., Chem. Sci. Trans., 2013, vol. 2, pp. 1056–1062. https://doi.org/10.7598/cst2013.490
Rao, G.M., Reddy, Y.N., and Kumar, B.V., Int. J. Appl. Biol. Pharm. Technol., 2013, vol. 4, pp. 38–46.
Kumar, B.V.S., Vaidya, S.D., Kumar, R.V., Bhirud, S.B., and Mane, R.B., J. Med. Chem., 2006, vol. 41, pp. 599–604. https://doi.org/10.1016/j.ejmech.2006.01.006
Gowda, N.R.T., Kavitha, C.V., Chiruvella, K.K., Joy, O., Rangappa, K.S., and Raghavan, S.C. Bioorg. Med. Chem. Lett., 2009, vol. 19, pp. 4594–4600. https://doi.org/10.1016/j.bmcl.2009.06.103
Tewari, A.K. and Mishra, A., Indian J. Chem., 2006, vol. 45, pp. 489–493.
Kerimov, I., Ayhan-Kilcigil, G., Can-Eke, B., Altanlar, N., and İscan, M., Enzyme Inhib. Med. Chem., 2007, vol. 22, pp. 696–701. https://doi.org/10.1080/14756360701228558
Yar, M.S., World Acad. Sci. Eng. Technol., 2009, vol. 55, pp. 593–599.
Sawant, R.L. and Kawade, D., Acta Pharmacol., 2011, vol. 61, pp. 353–361.
Mahajan, S.S. and Nandre, R.G., Indian J. Chem., 2006, vol. 45, pp. 1756–1758.
Hassan, Kh.M., Kamal El-Dean, A.M., Youssef, M.S.K., Atta, F.M., and Abbady, M.S., Phosphorus, Sulfur Silicon Relat. Elem., 1990, vol. 47, pp. 181–189. https://doi.org/10.1080/10426509008046859
Bakhite, E.A., Abd-Ella, A.A., El-Sayed, M.E.A., and Abdel-Raheem, Sh.A.A., J. Saudi Chem. Soc., 2017, vol. 21, pp. 95–104. https://doi.org/10.1016/j.jscs.2016.02.005
Attaby, F.A., Eldin, S.M., Basouni, W.M., and Elneairy, M.A.A., Phosphorus, Sulfur Silicon, 1996, vol. 108, pp. 31–39. https://doi.org/10.1080/10426509608029635
Hindler, J.A., Howard, B.J, and Keiser, J.F., Antimicrobial agents and susceptibility testing, in Clinical and Pathogenic Microbiology, Howard, B.J., Ed., St. Louis, MO, USA: Mosby-Year Book Inc., 1994.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ibrahim, O.F., Bakhite, E.A., Metwally, S.A. et al. Synthesis, Characterization, and Antifungal Activity of Some New Thieno[2,3-b]pyridines Incorporating Quinazoline or Benzimidazole Moiety. Russ J Bioorg Chem 47, 918–928 (2021). https://doi.org/10.1134/S1068162021040117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021040117