Abstract
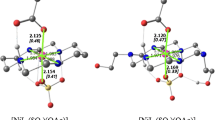
The formal potential of the couple of nonoxygenated U5+/U4+ ions bound in complexes with unsaturated heteropoly anions (HPAs) P2W17O 10−61 (I) and SiW11 8−O39 (II) in 0–1 M NaNO3 and 1 M (NaCl + HCl) in the range of pH 0.7–4.7 was measured. In 1 M NaNO3 solutions at pH 4.7–3.0 for I and 4.3–3.9 for II, the formal potentials are constant: 0.820 and 0.730 V, respectively. They preserve approximately the same value with a decrease in pH to 0.7 in 1 M (NaCl + HCl). The potential noticeably decreases with a decrease in the NaNO3 or NaCl concentration from 1 M to 0 (pH 4.1–4.7): to 0.09 and 0.05–0.06 V for I and II, respectively. Approximate constancy of the potential of the U5+/U4+ couple with a decrease in pH to 1 and lower distinguishes this couple from the M4+/M3+ couples (M = Ce, Am, Bk) whose potential appreciably grows with increasing acidity. This is due to the fact that the U5+ and M4+ ions in acid solutions remain in the form of complexes with the ratio M: HPA = 1: 2, whereas the M3+ ions pass into the form of 1: 1 complexes. Thus, variation of the formal potentials of all the Mn + 1/Mn+ couples in the presence of H+ and Na+ ions is associated with variation of the stability constants of the complexes M(HPA)2, which, in turn, is caused by interaction of single-charged ions with HPA. However, the H+ and Na+ ions interact with HPA by different mechanisms and therefore affect the potential of the U5+/U4+ couple differently.
Similar content being viewed by others
References
Termes, S.C. and Pope, M.T., Transition Met. Chem., 1978, vol. 3, pp. 103–108.
Maslov, L.P., Sirotinkina, L.V., and Rykov, A.G., Radiokhimiya, 1985, vol. 27, no. 6, pp. 732–736.
Chiang, M.-H., Soderholm, L., and Antonio, M., Eur. J. Inorg. Chem., 2003, no. 16, pp. 2929–2936.
Haraguchi, N., Okaue, Y., Isobe, T., and Matsuda, Y., Inorg. Chem., 1994, vol. 33, no. 6, pp. 1015–1020.
Baranov, A.A., Simakin, G.A., Kosyakov, V.N., et al., Radiokhimiya, 1981, vol. 23, no. 1, pp. 127–129.
Shilov, V.P., Yusov, A.B., Sokolova, M.N., and Fedoseev, A.M., Radiokhimiya, 2008, vol. 50, no. 2, pp. 203–208.
Tourné, C. and Tourné, G., Bull. Soc. Chim. Fr., 1969, no. 4, pp. 1124–1136.
Shilov, V.P., Yusov, A.B., and Fedoseev, A.M., Radiokhimiya, 2007, vol. 49, no. 4, pp. 318–322.
Bion, L., Moisy, Ph., and Madic, C., Radiochim. Acta, 1995, vol. 69, no. 4, pp. 251–257.
Mudgley, D. and Torrance, K., Potentiometric Water Analysis, Chichester: Wiley, 1978.
Contant, R. and Ciabrini, J.-P., J. Chem. Res. (S), 1982, nos. 3–7, pp. 641–660; J. Chem. Res. (M), 1982, no. 2, pp. 50–51.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.P. Shilov, A.B. Yusov, A.M. Fedoseev, Ph. Moisy, 2008, published in Radiokhimiya, 2008, Vol. 50, No. 5, pp. 393–396.
Rights and permissions
About this article
Cite this article
Shilov, V.P., Yusov, A.B., Fedoseev, A.M. et al. Influence of conditions on the potential of the couple of nonoxygenated U5+/U4+ ions bound in complexes with the P2W17O 10−61 and SiW11O 8−39 anions. Radiochemistry 50, 455–459 (2008). https://doi.org/10.1134/S1066362208050032
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362208050032