Abstract
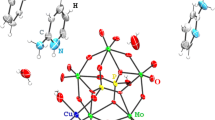
The structural features of the phases formed during crystallization of mixed copper and nickel complexes with nitrilotris(methylenephosphonic acid) [CuxNi(1 – x){N(CH2PO3)3}]Na4 · nH2O (x = 0–1) and the character of the M ← O(P) coordination bond have been investigated. The copper-dominant phase [(Cu,Ni){N(CH2PO3)3}]Na4 · 13H2O (sp. gr. P\(\bar {1}\), Z = 2, a = 10.0096(2)–10.0118(2) Å, b = 11.0311(3)–11.0330(2) Å, c = 12.2893(2)–12.3038(3) Å, α = 84.7180(10)°–84.785(2)°, β = 79.504(2)°–79.544(2)°, γ = 66.971(2)°–67.058(2)°) is characterized by the trigonal bipyramidal coordination of the metal atom (oxygen atoms of three different PO3 groups of the ligand molecule lie in the pyramid-base plane, and a nitrogen atom and the oxygen atom of the neighboring ligand molecule occupy opposite vertices). The nickel-containing phase [Ni(H2O){N(CH2PO3)3}]Na4 · 11H2O (sp. gr. C2/c, Z = 4, a = 11.9924(2)–12.05510(10) Å, b = 18.6049(3)–18.7152(2) Å, c = 21.0724(4)–21.1266(2) Å, β = 104.096(2)°–104.4960(10)°) is characterized by octahedral coordination of the Ni atom (oxygen atoms of different PO3 groups of the ligand molecule are located at three meridian octahedron vertices, and a nitrogen atom, a water molecule, and the oxygen atom of the neighboring ligand molecule occupy the rest three vertices). The dependence of the interatomic distances and bond angles in the coordination sphere of the metal atom on the Cu : Ni ratio has been studied. It is shown that the transition from the trigonal bipyramidal coordination to the octahedral coordination is accompanied by a sharp increase in the ionicity of the M ← O(P) coordination bond.






Similar content being viewed by others
REFERENCES
A. Cabeza, X. Ouyang, C. V. K. Sharma, et al., Inorg. Chem. 41, 2325 (2002). https://doi.org/10.1021/ic0110373
K. D. Demadis, S. D. Katarachia, and M. Koutmos, Inorg. Chem. Commun. 8, 254 (2005). https://doi.org/10.1016/j.inoche.2004.12.019
L. Cunha-Silva, L. Mafra, D. Ananias, et al., Chem. Mater. 19, 3527 (2007). https://doi.org/10.1021/cm070596q
M. Bazaga-García, G. K. Angeli, K. E. Papathanasiou, et al., Inorg. Chem. 55, 7414 (2016). https://doi.org/10.1021/acs.inorgchem.6b00570
N. V. Somov and F. F. Chausov, Crystallogr. Rep. 59 (1), 66 (2014).
N. V. Somov and F. F. Chausov, Crystallogr. Rep. 60 (2), 210 (2015).
N. V. Somov, F. F. Chausov, R. M. Zakirova, and I. V. Fedotova, Crystallogr. Rep. 61 (2), 216 (2016).
N. V. Somov, F. F. Chausov, R. M. Zakirova, and I. V. Fedotova, Russ. J. Coord. Chem. 41 (12), 798 (2015).
N. V. Somov, F. F. Chausov, R. M. Zakirova, et al., Crystallogr. Rep. 62 (6), 857 (2017).
N. V. Somov, F. F. Chausov, N. V. Lomova, et al., Russ. J. Coord. Chem. 43 (9), 583 (2017).
N. V. Somov, F. F. Chausov, R. M. Zakirova, et al., Russ. J. Coord. Chem. 43 (12), 864 (2017).
N. V. Somov, F. F. Chausov, and R. M. Zakirova, Crystallogr. Rep. 61 (3), 395 (2016).
N. V. Somov, F. F. Chausov, and R. M. Zakirova, Crystallogr. Rep. 61 (4), 606 (2016).
N. V. Somov, F. F. Chausov, R. M. Zakirova, et al., Russ. J. Coord. Chem. 43 (6), 373 (2017).
N. V. Somov, F. F. Chausov, R. M. Zakirova, et al., Crystallogr. Rep. 63 (6), 901 (2018).
N. V. Somov, F. F. Chausov, R. M. Zakirova, et al., Crystallogr. Rep. 63 (3), 364 (2018).
Yu. I. Kuznetsov, Usp. Khim. 73 (1), 79 (2004).
Yu. I. Kuznetsov, Fizikokhim. Poverkhn. Zashch. Mater. 38 (2), 122 (2002).
N. V. Lomova, F. F. Chausov, and I. N. Shabanova, Bull. Russ. Acad. Sci.: Phys. 82 (7), 884 (2018).
N. M. Dyatlova, V. Ya. Temkina, and K. I. Popov, Complexons and Complexonates of Metals (Khimiya, Moscow, 1988) [in Russian].
J. M. Stewart and E. C. Lingafelter, Acta Crystallogr. 12 (11), 842 (1959). https://doi.org/10.1107/s0365110x59002444
J. C. McDonald, T.-J. M. Luo, and G. T. R. Palmore, Cryst. Growth Des. 4 (6), 1203 (2004). https://doi.org/10.1021/cg049974j
L. Mao, S. J. Rettig, and R. C. Et al. Thompson, Can. J. Chem. 74, 433 (2011). https://doi.org/10.1139/v96-047
I. B. Bersuker, Electronic Structure and Properties of Coordination Compounds: Introduction into Theory (Khi-miya, Leningrad, 1976) [in Russian].
C. J. Ballhausen, Introduction to Ligand Field Theory (McGraw-Hill, New York, 1962).
F. F. Chausov, N. V. Lomova, N. V. Somov, et al., J. Cryst. Growth 524, 125187 (2019). https://doi.org/10.1016/j.jcrysgro.2019.125187
Rigaku Oxford Diffraction, CrysAlis PRO (Rigaku Oxford Diffraction, Yarnton, Oxfordshire, England, 2016).
G. M. Sheldrick, Acta Crystallogr. A 64, 112 (2008). https://doi.org/10.1107/S0108767307043930
L. J. Farrugia, J. Appl. Crystallogr. 32, 837 (1999). https://doi.org/10.1107/S0021889899006020
K. Momma and F. Izumi, J. Appl. Crystallogr. 44, 1272 (2011). https://doi.org/10.1107/S0021889811038970
E. N. Sviridova and N. V. Ivanova, Vestn. KemGU 3 (3), 108 (2013).
V. A. Trapeznikov, I. N. Shabanova, A. V. Kholzakov, and A. G. Ponomaryov, J. Electron Spectrosc. Relat. Phenom. 137, 383 (2004). https://doi.org/10.1016/j.elspec.2004.02.115
M. Wojdyr, J. Appl. Crystallogr. 43, 1126 (2010). https://doi.org/10.1107/S0021889810030499
J. J. Daly and P. J. Wheatley, J. Chem. Soc. A 212 (1967). https://doi.org/10.1039/J19670000212
A. W. Addison, T. N. Rao, J. Reedijk, et al., J. Chem. Soc., Dalton Trans., No. 7, 1349 (1984). https://doi.org/10.1039/dt9840001349
N. V. Somov and P. V. Andreev, Crystallogr. Rep. 63 (1), 32 (2018). https://doi.org/10.1134/S1063774518010170
R. D. Shannon, Acta Crystallogr. A 32, 751 (1976). https://doi.org/10.1107/S0567739476001551
C. A. Tolman, W. M. Riggs, W. J. Linn, et al., Inorg. Chem. 12 (12), 2770 (1973). https://doi.org/10.1021/ic50130a006
T. Yoshida and K. Yamasaki, Bull. Chem. Soc. Jpn. 54 (3), 935 (1981). https://doi.org/10.1246/bcsj.54.935
P. Brand and H. Freiser, Analyt. Chem. 46 (8), 1147 (1974). https://doi.org/10.1021/ac60344a010
T. Yoshida, K. Yamasak, and S. Sawada, Bull. Chem. Soc. Jpn. 51 (5), 1561 (1978). https://doi.org/10.1246/bcsj.51.1561
B. Cordero, V. Gómez, A. E. Platero-Prats, et al., Dalton Trans. 2832 (2008). https://doi.org/10.1039/b801115j
Funding
This study was performed within the basic part of a state contract (project no. 3.6502.2017/BCh) for higher educational institutions and scientific organizations in the part concerning the XRD analysis. The spectroscopic analysis was performed within a State assignment of the Ministry of Science and Higher Education of the Russian Federation (state registration no. АААА-А19-119093090055-2) using equipment of the Shared Research Center “Center of Physical and Physicochemical Methods for Analysis and Study of the Properties and Characteristics of Surfaces, Nanostructures, Materials, and Products” of the Udmurt Federal Research Center (Ural Branch, Russian Academy of Sciences), supported by the Ministry of Science and Higher Education of the Russian Federation within the Federal Target Program “Research and Development in the Priority Fields of the Scientific and Technological Complex of Russia for 2014–2020” (project no. RFMEFI62119X0035).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by Yu. Sin’kov
Rights and permissions
About this article
Cite this article
Somov, N.V., Chausov, F.F., Lomova, N.V. et al. The Crystal-Chemical Features of Phases and the Nature of the Coordination Bond in the System [CuxNi(1 – x){N(CH2PO3)3}]Na4 · nH2O (x = 0–1). Crystallogr. Rep. 65, 726–739 (2020). https://doi.org/10.1134/S1063774520050211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774520050211