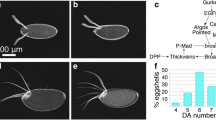
Great was the one who gave the direction.
Friedrich Wilhelm Nietzsche.
Abstract
Morphogenetic movement of cells is of significant importance in embryogenesis. It is necessary to identify the final position and configuration of the embryo’s tissues and share the positional information in differentiation. The healing of tissular injuries happens in the adult organism due to the cell layers’ movement, and the directed macrophages migration to the nidus of infection assists the neutralization of inflammatory processes. Owing to the good level of knowledge, the fruit fly D. melanogaster is a perfect model object for the study of morphogenetic events in embryogenesis in connection with the cells movement. The description of Drosophila embryogenesis, mechanisms of ventral furrow formation, elongation of the germ band, and its contraction accompanied by dorsal closure was given. During these processes the movement of epithelial cells and the entire epithelial strata happens. The information about the genetic regulation of morphogenetic movement of D. melanogaster taking into consideration the scale of evolutionary conservatism of the cascades of the main signal, which control these processes in vertebrates, is presented.
Similar content being viewed by others
References
Adler, P.N., Planar Signaling and Morphogenesis in Drosophila, Devel. Cell, 2002, vol. 2, pp. 525–535.
Axelrod, J.D., Unipolar Membrane Association of Dishevelled Mediates Frizzled Planar Cell Polarity Signaling, Genes Devel., 2001, vol. 15, pp. 1182–1187.
Bastock, R., Strutt, H., and Strutt, D., Strabismus is Asymmetrically Localised and Binds to Prickle and Dishevelled during Drosophila Planar Polarity Patterning, Development, 2003, vol. 130, pp. 3007–3014.
Baum, B. and Perrimon, N., Spatial Control of the Actin Cytoskeleton in Drosophila Epithelial Cells, Nat. Cell Biol., 2001, vol. 3, pp. 883–890.
Blankenship, J.T., Backovic, S., Sanny, J.S.P., et al., Multicellular Rosette Formation Links Planar Cell Polarity to Tissue Morphogenesis, Devel. Cell, 2006, vol. 11, pp. 459–470.
Brouns, M.R., Matheson, S.F., Hu, K.Q., et al., The Adhesion Signaling Molecule P190 RhoGAP is Required for Morphogenetic Processes in Neural Development, Development, 2000, vol. 127, pp. 4891–4903.
Campos-Ortega, J.A. and Hartenstein, V., The Embryonic Development of Drosophila Melanogaster, Berlin: Springer, 1985.
Chong, J.A., Moran, M.M., Teichmann, M., et al., TATA-Binding Protein (TBP)-Like Factor (TLF) is a Functional Regulator of Transcription: Reciprocal Regulation of the Neurofibromatosis type 1 and c-fos Genes by TLF/TRF2 and TBP, Mol. Cell. Biol., 2005, vol. 25, pp. 2632–2643.
Ciruna, B., Jenny, A., Lee, D., et al., Planar Cell Polarity Signalling Couples Cell Division and Morphogenesis During Neurulation, Nature, 2006, vol. 439, pp. 220–224.
Colas, J.F., Launay, J.-M., and Maroteaux, L., Maternal and Zygotic Control of Serotonin Biosynthesis are Both Necessary for Drosophila Germband Extension, Mech. Devel., 1999a, vol. 87, pp. 67–76.
Colas, J.F., Launay, J.M., Kellermann, O., et al., Drosophila 5-HT2 Serotonin Receptor: Coexpression with Fushi-Tarazu during Segmentation, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, pp. 5441–5445.
Colas, J.F., Launay, J.-M., Vonesch, J.-L., et al., Serotonin Synchronises Convergent Extension of Ectoderm with Morphogenetic Gastrulation Movements in Drosophila, J. High Resolut. Chromatogr. Chromatogr. Commun., 1999b, vol. 87, pp. 77–91.
Costa, M., Wilson, E.T., and Wieschaus, E., A Putative Cell Signal Encoded by the Folded Gastrulation Gene Coordinates Cell Shape Changes during Drosophila Gastrulation, Cell, 1994, vol. 76, pp. 1075–1089.
Das, G., Jenny, A., Klein, T.J., et al., Diego Interacts with Prickle and Strabismus/Van Gogh to Localize Planar Cell Polarity Complexes, Development, 2004, vol. 131, pp. 4467–4476.
Dawes-Hoang, R.E., Parmar, K.M., Christiansen, A.E., et al., Folded Gastrulation, Cell Shape Change and the Control of Myosin Localization, J. High Resolut. Chromatogr. Chromatogr. Commun., 2005, vol. 132, pp. 4165–4178.
Fehon, R.G., Dawson, I.A., and Artavanis-Tsakonas, S.A., Drosophila Homologue of Membrane-Skeleton Protein 4.1 is Associated with Septate Junctions and is Encoded by the Coracle Gene, J. High Resolut. Chromatogr. Chromatogr. Commun., 1994, vol. 120, pp. 545–557.
Fischer, E. and Legue, E., Doyen A, Et Al. Defective Planar Cell Polarity in Polycystic Kidney Disease, Nat. Genet., 2006, vol. 38, pp. 21–23.
Foe, V.E. and Alberts, B.M., Studies of Nuclear and Cytoplasmic Behaviour during the Five Mitotic Cycles That Precede Gastrulation in Drosophila Embryogenesis, J. Cell Sci., 1983, vol. 61, pp. 31–70.
Fox, D.T. and Peifer, M., Abelson Kinase (Abl) and RhoGEF2 Regulate Actin Organization during Cell Constriction in Drosophila, Development, 2007, vol. 134, pp. 567–578.
Gertler, F.B., Comer, A.R., Juang, J.L., et al., Enabled, a Dosage-Sensitive Suppressor of Mutations in the Drosophila Abl Tyrosine Kinase, Encodes an Abl Substrate with SH3 Domain-Binding Properties, Genes Devel., 1995, vol. 9, pp. 521–533.
Ghabrial, A.S. and Krasnow, M.A., Social Interactions among Epithelial Cells during Tracheal Branching Morphogenesis, Nature, 2006, vol. 441, pp. 746–749.
Glise, B. and Nosell, S., Coupling of Jun Amino-Terminal Kinase and Decapentaplegic Signaling Pathways in Drosophila Morphogenesis, Genes Devel., 1997, vol. 11, pp. 1738–1747.
Glise, B., Bourbon, H., and Noselli, S., Hemipterous Encodes a Novel Drosophila MAP Kinase Kinase, Required for Epithelial Cell Sheet Movement, Cell, 1995, vol. 83, pp. 451–461.
Goldstein, E.S., Treadway, S.L., Stephenson, A.E., et al., A Genetic Analysis of the Cytological Region 46C-F Containing the Drosophila melanogaster Homolog of the Jun Proto-Oncogene, Mol. Genet. Genomics, 2001, vol. 266, pp. 695–700.
Grevengoed, E.E., Loureiro, J.J., Jesse, T.L., et al., Abelson Kinase Regulates Epithelial Morphogenesis in Drosophila, J. Cell Biol., 2001, vol. 155, pp. 1185–1198.
Gubb, D. and Garcia-Bellido, A., A Genetic Analysis of the Determination of Cuticular Polarity during Development in Drosophila melanogaster, J. Embryol. Exp. Morphol., 1982, vol. 62, pp. 37–57.
Hacker, U. and Perrimon, N., DRhoGEF2 Encodes a Member of the Dbl Family of Oncogenes and Controls Cell Shape Changes during Gastrulation in Drosophila, Genes Devel., 1998, vol. 12, pp. 274–284.
Hartenstein, V., Atlas of Drosophila Development, New York: Cold Spring Harbor Lab. Press, 1993.
Hyodo-Miura, J., Yamamoto, T.S., Hyodo, A.C., et al., XGAP, an ArfGAP, is Required for Polarized Localization of PAR Proteins and Cell Polarity in Xenopus Gastrulation, Devel. Cell, 2006, vol. 11, pp. 69–79.
Irvine, K. and Wieschaus, E., Cell Intercalation during Drosophila Germband Extension and Its Regulation by Pair-Rule Segmentation Genes, Development, 1994, vol. 120, pp. 827–841.
Ivanova-Kazas, O.M. and Krichinskaya, E.B., Kurs sravnitel’noi embriologii bespozvonochnykh zhivotnykh (A Course of Comparative Embryology of Invertebrates), Leningrad: Leningr. Gos. Univ., 1988.
Jiang, L. and Crews, S.T., Transcriptional Specificity of Drosophila Dysfusion and the Control of Tracheal Fusion Cell Gene Expression, J. Biol. Chem., 2007, vol. 282, pp. 28659–28668.
Jones, C. and Chen, P., Planar Cell Polarity Signaling in Vertebrates, BioEssays, 2007, vol. 29, pp. 120–132.
Jürgens, G., Wieschaus, E., Nusslein-Volhard, C., et al., Mutations Affecting the Pattern of the Larval Cuticle in Drosophila melanogaster: II. Zygotic Loci on the Third Chromosome, Roux’s Arch. Devel. Biol., 1984, vol. 193, pp. 283–295.
Kaltschmidt, J.A., Lawrence, N., Morel, V., et al., Planar Polarity and Actin Dynamics in the Epidermis of Drosophila, Nature Cell Biol., 2002, vol. 4, pp. 937–944.
Keller, R., Davidson, L., Edlund, A., et al., Mechanisms of Convergence and Extension by Cell Intercalation, Philos. Trans. R. Soc. L. B. Biol. Sci., 2000, vol. 355, pp. 897–922.
Keller, R., Shaping the Vertebrate Body Plan by Polarized Embryonic Cell Movements, Science, 2002, vol. 298, pp. 1950–1954.
Knust, E., Drosophila Morphogenesis: Follow-My-Leader in Epithelia, Curr. Biol., 1996, vol. 6, pp. 379–381.
Koleske, A.J., Gifford, A.M., Scott, M.L., et al., Essential Roles for the Abl and Arg Tyrosine Kinases in Neurulation, Neuron, 1998, vol. 21, pp. 1259–1272.
Kuzin, B., Regulski, M., Stasiv, Y., et al., Nitric Oxide Interacts with Retinoblastoma Pathway to Control Eye Development in Drosophila, Curr. Biol., 2000, vol. 10, pp. 459–462.
Kuzin, B., Roberts, I., Peunova, N., and Enikolopov, G., Nitric Oxide Regulates Cell Proliferation during Drosophila Development, Cell, 1996, vol. 87, pp. 639–649.
Lanier, L.M. and Gertler, F.B., From Abl to Actin: Abl Tyrosine Kinase and Associated Proteins in Growth Cone Motility, Curr. Opin. Neurobiol., 2000, vol. 10, pp. 80–87.
Lawrence, P.A., Casal, J., and Struhl, G., Towards a Model of the Organisation of Planar Polarity and Pattern in the Drosophila Abdomen, Development, 2002, vol. 129, pp. 2749–2760.
Logan, C.Y. and Nusse, R., The Wnt Signaling Pathway in Development and Disease, Annu. Rev. Cell Devel. Biol., 2004, vol. 20, pp. 781–810.
Luschnig, S., Moussian, B., Krauss, J., et al., An F1 Genetic Screen for Maternal-Effect Mutations Affecting Embryonic Pattern Formation in Drosophila melanogaster, Genetics, 2004, vol. 167, pp. 325–342.
MacKrell, A.J., Blumberg, B., Haynes, S.R., et al., The Lethal Myospheroid Gene of Drosophila Encodes a Membrane Protein Homologous to Vertebrate Integrin Subunits, Proc. Natl. Acad. Sci. USA, 1988, vol. 85, pp. 2633–2637.
Marshall, C.J., MAP Kinase Kinase Kinase, MAP Kinase Kinase and MAP Kinase, Curr. Opin. Genet. Devel., 1994, vol. 4, pp. 82–89.
Martin, D., Zusman, S., Li, X., et al., Wing Blister, a New Drosophila Laminin Alpha Chain Required for Cell Adhesion and Migration during Embryonic and Imaginal Development, J. Cell Biol., 1999, vol. 145, pp. 191–201.
Martin, P. and Nobes, C.D., An Early Molecular Component of the Wound Healing Process in Rat Embryos: Induction of c-fos Protein in Cells at the Epidermal Wound Margin, Mech. Devel., 1992, vol. 38, pp. 209–215.
Martin, P., Dickson, M.C., Milan, F.A., et al., Rapid Induction and Clearance of TGFβ-1 is an Early Response to Wounding in the Mouse Embryo, Devel. Genet., 1993, vol. 14, pp. 225–238.
Martin, P., Wound Healing—Aiming for Perfect Skin Regeneration, Science, 1997, vol. 276, pp. 75–81.
Miller, J.R., Rowning, B.A., Larabell, C.A., et al., Establishment of the Dorsal-Ventral Axis in Xenopus Embryos Coincides with the Dorsal Enrichment of Dishevelled that is Dependent on Cortical Rotation, J. Cell Biol., 1999, vol. 146, pp. 427–437.
Mlodzik, M., Planar Cell Polarization: do the Same Mechanisms Regulate Drosophila Tissue Polarity and Vertebrate Gastrulation?, Trends Genet., 2002, vol. 18, pp. 564–571.
Modestova, E.A., Kopytova, D.V., Georgieva, S.G., and Simonova, O.B., P-Ph-Mediated Repression of the legarista-wing complex Gene Transcription in Drosophila, Genetika, 2003, vol. 39, no. 5, pp. 713–716.
Modestova, E.A., Vorontsova, Yu.E., Korochkin, L.I., and Simonova, O.B., Induction of Lethal Mutations of the Legarista-wing complex Gene of Drosophila melanogaster, Dokl. Akad. Nauk, 2005, vol. 403, no. 4, pp. 564–565. [Dokl. (Engl. Transl.), vol. 403, no. 4, pp. 282–283].
Morize, P., Christiansen, A.E., Costa, M., et al., Hyperactivation of the Folded Gastrulation Pathway Induces Specific Cell Shape Changes, Development, 1998, vol. 125, pp. 589–597.
Nelson, W.J., Tube Morphogenesis: Closure, But Many Openings Remain, Trends Cell Biol., 2003, vol. 13, pp. 615–621.
Nikolaidou, K.K. and Barrett, K., A Rho GTPase Signaling Pathway is Used Reiteratively in Epithelial Folding and Potentially Selects the Outcome of Rho Activation, Curr. Biol., 2004, vol. 14, pp. 1822–1826.
Ninomiya, H., Elinson, R.P., and Winklbauer, R., Antero-Posterior Tissue Polarity Links Mesoderm Convergent Extension to Axial Patterning, Nature, 2004, vol. 430, pp. 364–367.
Nobes, C.D. and Hall, A., Rho GTPases Control Polarity, Protrusion, and Adhesion during Cell Movement, J. Cell Biol., 1999, vol. 144, pp. 1235–1244.
Oda, H., Tsukita, S., and Takeichi, M., Dynamic Behavior of the Cadherin-Based Cell-Cell Adhesion System during Drosophila Gastrulation, Devel. Biol., 1998, vol. 203, pp. 435–450.
Parks, S. and Wieschaus, E., The Drosophila Gastrulation Gene Concertina Encodes a G Alpha-Like Protein, Cell, 1991, vol. 64, pp. 447–458.
Pastor-Pareja, J.C., Grawe, F., Martin-Blanco, E., et al., Invasive Cell Behavior during Drosophila Imaginal Disc Eversion is Mediated by the JNK Signaling Cascade, Devel. Cell, 2004, vol. 7, pp. 387–399.
Perkins, K.K., Admon, A., Patel, N., et al., The Drosophila Fos-Related AP-1 Protein Is a Developmentally Regulated Transcription Factor, Genes Devel., 1990, vol. 4, pp. 822–834.
Perrimon, N. and Mahowald, A., Multiple Functions of Segment Polarity Genes in Drosophila, Devel. Biol, 1987, vol. 119, pp. 587–600.
Peunova, N., Sheinker, V., Ravi, K., and Enikolopov, G., Nitric Oxide Coordinates Cell Proliferation and Cell Movements during Early Development of Xenopus, Cell Cycle, 2007, vol. 6, pp. 3132–3144.
Pilot, F. and Lecuit, T., Compartmentalized Morphogenesis in Epithelia: from Cell to Tissue Shape, Devel. Dyn., 2005, vol. 232, pp. 685–694.
Poluektova, E.V., Mitrofanov, V.G., Burychenko, G.M., et al., Ob“ekty biologii razvitiya. Drozofila — Drosophila (Developmental Biology Objects: Drosophila), Moscow: Nauka, 1975.
Riesgo-Escovar, J.R. and Hafen, E., Drosophila Jun Kinase Regulates Expression of Decapentaplegic via the ETSDomain Protein Aop and the AP-1 Transcription Factor, DJun, during Dorsal Closure, Genes Devel., 1997, vol. 11, pp. 1717–1727.
Ring, J.M. and Martinez-Arias, A., Puckered, a Gene Involved in Position-Specific Cell Differentiation in the Dorsal Epidermis of the Drosophila Larva, Development, 1993, vol. 119, pp. 251–259.
Rogers, S.L., Wiedemann, U., Hacker, U., et al., Drosophila RhoGEF2 Associates with Microtubule Plus Ends in an EB1-Dependent Manner, Curr. Biol., 2004, vol. 14, pp. 1827–1833.
Schaerlinger, B., Launay, J.M., Vonesch, J.L., et al., Gain of Affinity Point Mutation in the Serotonin Receptor Gene 5-HT2Dro Accelerates Germband Extension Movements during Drosophila Gastrulation, Devel. Dyn., 2007, vol. 236, pp. 991–999.
Schock, F. and Perrimon, N., Cellular Processes Associated with Germ Band Retraction in Drosophila, Devel. Biol., 2002, vol. 248, pp. 29–39.
Schock, F. and Perrimon, N., Retraction of the Drosophila Germ Band Requires Cell-Matrix Interaction, Genes Devel., 2003, vol. 17, pp. 597–602.
Schoenwolf, G.C. and Alvarez, I.S., Roles of Neuroepithelial Cell Rearrangement and Division in Shaping of the Avian Neural Plate, Development, 1989, vol. 106, pp. 427–439.
Shimada, Y., Yonemura, S., Ohkura, H., et al., Polarized Transport of Frizzled Along the Planar Microtubule Arrays in Drosophila Wing Epithelium, Devel. Cell, 2006, vol. 10, pp. 209–222.
Simonova, O.B., Kuzin, B.A., Georgiev, P.G., and Gerasimova, T.I., A New Regulatory Mutation of Drosophila, Genetika, 1992, vol. 28, no. 2, pp. 164–167.
Simonova, O.B., A New trans-Regulatory Locus of Drosophila, Genetika, 2000, vol. 36, no. 11, pp. 1464–1474.
Slezinger, M.S. and Kuzin, B.A., Nitric Oxide Synthase Mediates Regulation of Cell Polarity and Movement during Drosophila melanogaster Morphogenesis, Ontogenez, 2009, vol. 40, no. 1, pp. 40–47 [Rus. J. Dev. Biol., vol. 40, no. 1, pp. 31–37].
Stasiv, Y., Kuzin, B., Regulski, M., et al., Regulation of Multimers via Truncated Isoforms: a Novel Mechanism to Control Nitric Oxide Signaling, Genes Devel., 2004, vol. 18, pp. 1812–1823.
Stasiv, Y., Regulski, M., Kuzin, B., et al., The Drosophila Nitric Oxide Synthase Gene (DNOS) Encodes a Family of Proteins that can Modulate NOS Activity by Acting as Dominant Negative Regulators, J. Biol. Chem., 2001, vol. 276, pp. 42241–42251.
Strutt, D.I., The Asymmetric Subcellular Localisation of Components of the Planar Polarity Pathway, Semin. Cell Devel. Biol, 2002, vol. 13, pp. 225–231.
Suzuki, A. and Ohno, S., The PARaPKC System: Lessons in Polarity, J. Cell Sci., 2006, vol. 119, pp. 979–987.
Tree, D.R., Shulman, J.M., Rousset, R., et al., Prickle Mediates Feedback Amplification to Generate Asymmetric Planar Cell Polarity Signaling, Cell, 2002, vol. 109, pp. 371–381.
Turner, F.R. and Mahowald, A.P., Scanning Electron Microscopy of Drosophila melanogaster Embryogenesis. II. Gastrulation and Segmentation, Devel. Biol., 1977, vol. 57, pp. 403–416.
Usui, T., Shima, Y., Shimada, Y., et al., Flamingo, a Seven-Pass Transmembrane Cadherin, Regulates Planar Cell Polarity Under the Control of Frizzled, Cell, 1988, vol. 98, pp. 585–595.
Vorontsova, Yu.G., Modestova, E.A., Burdina, N.V., and Simonova, O.B., Restoring Viability of Lethal Mutants for the leg-arista-wing-complex Gene in Rescue Experiments with Transgenic Constructs that Express the trf2 Gene Domains in Drosophila melanogaster, Dokl. Akad. Nauk, 2007, vol. 417, no. 1, pp. 133–135 [Dokl. (Engl. Transl.), vol. 417, no. 1, pp. 429–431].
Wong, L.L. and Adler, P.N., Tissue Polarity Genes of Drosophila Regulate the Subcellular Location for Prehair Initiation in Pupal Wing Cells, J. Cell Biol., 1993, vol. 123, pp. 209–221.
Young, P.E., Richman, A.M., Ketchum, A.S., et al., Morphogenesis in Drosophila Requires Nonmuscle Myosin Heavy Chain Function, Genes Devel., 1993, vol. 7, pp. 29–41.
Zallen, J.A. and Wieschaus, E., Patterned Gene Expression Directs Bipolar Planar Polarity in Drosophila, Devel. Cell, 2004, vol. 6, pp. 343–355.
Zecchini, V., Brennan, K., and Martinez-Arias, A., An Activity of Notch Regulates JNK Signalling and Affects Dorsal Closure in Drosophila, Curr. Biol., 1999, vol. 9, pp. 460–469.
Zeitlinger, J. and Bohmann, D., Thorax Closure in Drosophila: Involvement of Fos and the JNK Pathway, Development, 1999, vol. 126, pp. 3947–3956.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.B. Simonova, N.V. Burdina, 2009, published in Ontogenez, 2009, Vol. 40, No. 5, pp. 355–372.
Rights and permissions
About this article
Cite this article
Simonova, O.B., Burdina, N.V. Morphogenetic movement of cells in embryogenesis of Drosophila melanogaster: Mechanism and genetic control. Russ J Dev Biol 40, 283–299 (2009). https://doi.org/10.1134/S1062360409050038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360409050038