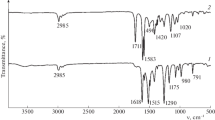
Abstract
Adsorption of some 1,3,4-oxadiazoles and 1,2,4,5-tetrazines from water–acetonitrile solutions on Luna C18 and Discovery C18 silica gels with grafted octadecyl groups has been studied in a wide range of compositions at temperatures of 313.15–333.15 K. The comparative analysis of the liquid-phase adsorption of the heterocycles has revealed a number of differences between the mechanisms of binding molecules of the studied compounds by the surfaces of these octadecyl-derivatives of silica gel. The log–log dependences of the retention factor on the molar fraction of acetonitrile in the solutions are strictly linear only for adsorption on Luna C18 silica gel, which is characterized by an increased hydrophobicity. For the adsorption of the studied heterocycles on Discovery C18 silica gel, the concentration dependences of the retention at low and high acetonitrile concentrations in the solution are described by quadratic functions and characterized by higher retention values as compared with those for the linear dependence because of the lower hydrophobicity of this silica gel. It has been found that the surface layer of Luna C18 modified silica gel is enriched with acetonitrile molecules to a higher extent than the surface layer of Discovery C18 silica gel. It has been shown that one adsorbed molecule of 1,3,4-oxadiazoles and 1,2,4,5-tetrazines displaces three and two molecules of pre-adsorbed acetonitrile from the surfaces of Luna C18 and Discovery C18 silica gels, respectively. Linear correlation equations have been proposed for calculating the retention of the studied heterocycles on Luna C18 and Discovery C18 silica gels from mobile phases of arbitrary compositions at different temperatures of chromatographic columns.





Similar content being viewed by others
REFERENCES
Žuvela, P., Skoczylas, M., Liu, J.J., Bączek, T., Kaliszan, R., Wong, M.W., and Buszewski, B., Chem. Rev., 2019, vol. 119, p. 3674.
Sandron, S., Rojas, A., Wilson, R., Davies, N.W., Haddad, P.R., Shellie, R.A., Nesterenko, P.N., Kelleher, B.P., and Paull, B., Environ. Sci.: Process. Impacts, 2015, vol. 17, p. 1531.
Janas, P., Bocian, S., Jandera, P., Kowalkowski, T., and Buszewski, B., J. Chromatogr. A, 2015, vol. 1429, p. 198.
Taraba, L., Kŕižek, T., Kozlik, P., Hodek, O., and Coufal, P., J. Sep. Sci., 2018, vol. 41, p. 2886.
Deineka, V.I., Kul’chenko, Ya.Yu., and Deineka, L.A., Russ. J. Phys. Chem. A, 2019, vol. 93, p. 572.
Wiczling, P., Kubik, L., and Kaliszan, R., Anal. Chem., 2015, vol. 87, p. 7241.
Sancho, M.I., Blanco, S.E., and Castro, E.A., J. Chem. Eng. Data, 2010, vol. 55, p. 4768.
Rehl, B., Li, Z., and Gibbs, J.M., Langmuir, 2018, vol. 34, p. 4445.
Nekrasova, N.A. and Kurbatova, S.V., Russ. J. Phys. Chem. A, 2019, vol. 93, p. 67.
Rybka, J., Holtzel, A., Steinhoff, A., and Tallarek, U., J. Phys. Chem. C, 2019, vol. 123, p. 3672.
Petrović, S.M. and Lomić, S.M., Chromatografia, 1989, vol. 27, p. 378.
Lee, C.S. and Cheong, W.J., J. Chromatogr. A, 1999, vol. 848, p. 9.
Kurbatova, S.V., Saifutdinov, B.R., Larionov, O.G., and Meshkovaya, V.V., Russ. J. Phys. Chem. A, 2009, vol. 83, p. 471.
Kurbatova, S.V. and Saifutdinov, B.R., Russ. J. Phys. Chem. A, 2009, vol. 83, p. 1223.
Saifutdinov, B.R., Kurbatova, S.V., and Emel’yanova, N.S., Russ. J. Phys. Chem. A, 2010, vol. 84, p. 673.
Shafigulin, R.V., Myakishev, A.A., Il’ina, E.A., Il’in, M.M., Davankov, V.A., and Bulanova, A.V., Russ. J. Phys. Chem. A, 2011, vol. 85, p. 1322.
Saifutdinov, B.R., Izv. Akad. Nauk, Ser. Khim., 2014, no. 12, p. 2609.
Polunin, K.E., Polunina, I.A., and Roldughin, V.I., Colloid J., 2015, vol. 77, p. 327.
Nekrasova, N.A. and Kurbatova, S.V., J. Chromatogr. A, 2017, vol. 1492, p. 55.
Karaseva, I.N., Karasev, M.O., Nechaeva, O.N., and Kurbatova, S.V., Russ. J. Phys. Chem. A, 2019, vol. 93, p. 160.
Visky, D., Neyden, Y.V., Ivanyi, T., Baten, P., Beer, J.D., Kovács, Z., Noszál, B., Dehouck, P., Roets, E., Massart, D.L., and Hoogmartens, J., J. Chromatogr. A, 2003, vol. 1012, p. 11.
Caiali, E., David, V., Aboul-Enein, H.Y., and Moldoveanu, S.C., J. Chromatogr. A, 2016, vol. 1435, p. 85.
Caiali, E., Moldoveanu, S.C., and David, V., Rev. Roum. Chim., 2017, vol. 62, p. 629.
www.phenomenex.com/Products/HPLCDetail/luna#.
www.supelco.com.tw/B-1%2013-66.pdf.
ACKNOWLEDGMENTS
We are grateful to Associate Professor of the Department of Organic Chemistry of Samara State Technical University A.V. Yudashkin for giving us the possibility to use heterocycles 1–5, which he has synthesized, and the Center for Collective Use of the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, for allowing us to use experimental equipment.
Funding
This work was carried out within the framework of a state order to the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
B.R. Saifutdinov is grateful to the Russian Foundation for Basic Research (project no. 17-03-01308-a), the Council for Grants of the President of the Russian Federation for State Support of Young Russian Scientists and State Support of Leading Scientific Schools of the Russian Federation (project no. MK-5757.2018.3) and the Ministry of Science and Higher Education of the Russian Federation (project no. 4.7150.2017/8.9) for partial financial support of the performed studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Saifutdinov, B.R., Buryak, A.K. On the Differences in the Mechanisms of Adsorption of Aromatic Heterocycles from Water–Acetonitrile Solutions on Octadecyl-Bonded Silica Gels. Colloid J 81, 555–562 (2019). https://doi.org/10.1134/S1061933X19050107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19050107