Abstract
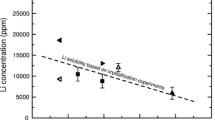
The data of experiments are presented on the Ta and Nb concentrations in acidic magma melts of various compositions upon dissolution of pyrochlore and microlite at 650‒850°C and 100‒400 MPa, which allow one to obtain quantitative characteristics to create physicochemical models of the genesis of Ta–Nb deposits. Upon the dissolution of pyrochlore in granitoid melts at 100 MPa, the maximum Nb content of 0.7‒1.9 wt % was found in alkaline solutions, with a decrease to 0.06–0.38 wt % and a moderate increase to 0.11–0.41 wt % in sub- and peraluminous melts, respectively. The growth in temperature increased the solubility of pyrochlore; the effect of an increase in pressure was ambiguous. Pyrochlore was unstable in peraluminous granite melts. Upon the dissolution of microlite, the Nb/Ta ratio in the melt was nearly doubled with an increase in the alumina content in the melt.




Similar content being viewed by others
REFERENCES
E. V. Badanina, L. F. Syritso, E. V. Volkova, R. Tomas, and R. B. Trumbull, Petrology 18 (2), 131–158 (2010).
S. M. Beskin, A. M. Grebennikov, and V. V. Matias, Petrologiya 2 (1), 68–87 (1994) [in Russian].
S. M. Beskin, V. E. Zagorskii, L. G. Kuznetsova, I. I. Kursinov, V. N. Pavlova, V. Yu. Prokof’ev, A. E. Tsyganov, and B. M. Shmakin, Geol. Rudn. Mestorozhd. 36 (4), 310–325 (1994) [in Russian].
G. P. Zaraiskii, V. Yu. Chevychelov, A. M. Aksyuk, V. S. Korzhinskaya, N. P. Kotova, A. F. Red’kin, and G. P. Borodulin, in Experimental Researches of Endogenous Processes. Commemorating Academician V. A. Zharikov (Inst. Probl. Chem. Phys. RAS, Chernogolovka, 2008), pp. 86–109 [in Russian].
V. F. Efimov, et al., Criteria for Prediction, Search and Promising Estimation of Deposits of Rare-Metal Granites of Alkaline-Earth Series. Methodological Recommendations, Ed. by E. K. Burenkov (Inst. Mineral., Geochem. Crystal Chem. Rare Elements, Moscow, 1992) [in Russian].
V. Yu. Chevychelov, Extended Abstract of Doctoral Dissertation in Geology and Mineralogy (Moscow, 2013) [in Russian].
V. Yu. Chevychelov, in Sofia Initiative “Mineral Variety Saving.” Proc. 8th Int. Symp. “Mineral Variety: Research and Saving” (Earth and Man National Museum, Sofia, 2016), pp. 22–34 [in Russian].
V. Yu. Chevychelov, G. P. Borodulin, and G. P. Zaraiskii, Geochem. Int. 48 (5), 456–464 (2010).
F. G. Reyf, R. Seltmann, and G. P. Zaraisky, Can. Mineral. 38, 915–936 (2000).
ACKNOWLEDGEMENTS
The authors are grateful to N.V. Chukanov for the samples of pyrochlore and microlite.
Funding
This study was supported by the Russian Foundation for Basic Research (project no. 18-05-01001A) and in part by the Institute of Experimental Mineralogy, Russian Academy of Sciences (project no. AAAA-A18-118020590151-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Rylova
Rights and permissions
About this article
Cite this article
Chevychelov, V.Y., Viryus, A.A. & Shapovalov, Y.B. Dissolution of Pyrochlore and Microlite in Alkaline, Subaluminous, and Peraluminous Granitoid Melts. Dokl. Earth Sc. 489, 1465–1468 (2019). https://doi.org/10.1134/S1028334X19120171
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X19120171