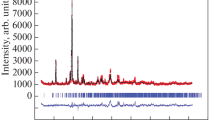
Abstract
The crystal structure of BiMnO3 + δ is studied as a function of the nominal oxygen content using X-ray synchrotron diffraction. It is found that an increase in the nominal oxygen-ion concentration leads to a series of phase transitions from the monoclinic (C2/c) to another monoclinic phase (P21/c) and then to the orthorhombic structure Pnma through the formation of a two-phase structural state. The indicated sequence of the phase transitions is accompanied by gradual destruction of the orbital ordering formed by the  orbitals of Mn3+ ions, which is caused by the nonuniform distribution of vacancies of manganese ions in the B positions of the perovskite lattice.
orbitals of Mn3+ ions, which is caused by the nonuniform distribution of vacancies of manganese ions in the B positions of the perovskite lattice.


Similar content being viewed by others
REFERENCES
C. Ederer and N. A. Spaldin, Phys. Rev. B 71, 060401(R) (2005). https://doi.org/10.1103/PhysRevB.71.060401
A. Moreira dos Santos, S. Parashar, A. R. Raju, Y. S. Zhao, A. K. Cheetham, and C. N. R. Rao, Solid State Commun. 122, 49 (2002). https://doi.org/10.1016/S0038-1098(02)00087-X
A. A. Belik, Adv. Mater. 12, 044610 (2011). https://doi.org/10.1088/1468-6996/12/4/044610
T. Kimura, S. Kawamoto, I. Yamada, M. Azuma, M. Takano, and Y. Tokura, Phys. Rev. B 67, 180401 (2003). https://doi.org/10.1103/PhysRevB.67.180401
T. Atou, H. Chiba, K. Ohoyama, Y. Yamaguchi, and Y. Syono, J. Solid State Chem. 145, 639 (1999). https://doi.org/10.1006/jssc.1999.8267
A. Moreira dos Santos, A. K. Cheetham, T. Atou, Y. Syono, Y. Yamaguchi, K. Ohoyama, H. Chiba, and C. N. R. Rao, Phys. Rev. B 66, 064425 (2002). https://doi.org/10.1103/PhysRevB.66.064425
A. A. Belik, S. Iikubo, T. Yokosawa, K. Kodama, N. Igawa, S. Shamoto, M. Azuma, M. Takano, K. Kimoto, Y. Matsui, and E. Takayama-Muromachi, J. Am. Chem. Soc. 129, 971 (2007). https://doi.org/10.1021/ja0664032
J. M. D. Coey, M. Viret, and S. von Molnár, Adv. Phys. 58, 567 (2009). https://doi.org/10.1080/00018730903303370
S. V. Trukhanov, V. A. Khomchenko, D. V. Karpinsky, M. V. Silibin, A. V. Trukhanov, L. S. Lobanovsky, H. Szymczak, C. E. Botez, and I. O. Troyanchuk, J. Rare Earths 37, 1242 (2019). https://doi.org/10.1016/j.jre.2018.12.010
J. B. Goodenough, A. Wold, R. J. Arnott, and N. Menyuk, Phys. Rev. 124, 373 (1961). https://doi.org/10.1103/PhysRev.124.373
I. V. Solovyev and Z. V. Pchelkina, New J. Phys. 10, 073021 (2008). https://doi.org/10.1088/1367-2630/10/7/073021
F. G. Figueiras, D. Karpinsky, P. B. Tavares, J. N. Gonçalves, et al., Phys. Chem. Chem. Phys. 19, 1335 (2017). https://doi.org/10.1039/c6cp07682c
H. Chiba, T. Atou, and Y. Syono, J. Solid State Chem. 132, 139 (1997). https://doi.org/10.1006/jssc.1997.7432
A. Sundaresan, R.V. K. Mangalam, A. Iyo, Y. Tanaka, and C.N.R. Rao, J. Mater. Chem. 18, 2191 (2008). https://doi.org/10.1039/b803118p
A. A. Belik, T. Kolodiazhnyi, Kosudac Kosuke, and Eiji Takayama-Muromachi, J. Mater. Chem. 19, 1593 (2009). https://doi.org/10.1039/b818645f
A. A. Belik, K. Kodama, N. Igawa, S. Shamoto, K. Kosuke, and E. Takayama-Muromachi, J. Am. Chem. Soc. 132, 8137 (2010). https://doi.org/10.1021/ja102014n
H. M. Rietveld, J. Appl. Crystallogr. 2, 65 (1969). https://doi.org/10.1107/S0021889869006558
J. Rodríguez-Carvajal, Phys. B (Amsterdam, Neth.) 192, 55 (1993). https://doi.org/10.1016/0921-4526(93)90108-I
ACKNOWLEDGMENTS
We thank the Helmholtz-Zentrum Berlin für Materialien und Energie for the synchrotron studies.
Funding
The research was carried out with the support of the Russian Foundation for Basic Research (project no. 20-52-00023) and the Belarusian Republican Foundation for Basic Research (project no. T20P-121) within the framework of the State assignment of the Ministry of Higher Education and Science, project FZWM-2020-0008.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
We declare that we have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sikolenko, V.V., Chobot, A.N., Bushinsky, M.V. et al. Crystal Structure and Orbital Ordering in BiMnO3 + δ (0 < δ ≤ 0.14) Ceramics. J. Surf. Investig. 16, 96–100 (2022). https://doi.org/10.1134/S1027451022010335
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451022010335