Abstract
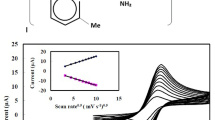
Co-deposited nickel–chromium (Ni–Cr) onto the glassy carbon electrode (GCE) is successfully used as new amperometric sensor for the determination of salicylic acid (SA). SA is detected by a surface catalyzed oxidation, involving nickel(III) oxyhydroxides in alkaline solution. The performance of the biosensor Ni–Cr/GCE is characterized by cyclic voltammetry, chrono-amperometry, scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) and X-ray diffraction (XRD). The electrochemical behavior of the Ni–Cr alloy is qualitatively similar to that of pure nickel electrode. However, it is proposed that a higher degree of disorder of the oxyhydroxide layer structure is present on the top of the alloy. The electroactivity of Ni–Cr/GCE is studied as a function of the molar fraction (Xf%) of Cr3+ in the deposition bath. The results show that Ni–Cr/GCE exhibits a high electrocatalytic activity for SA oxidation. The Ni–Cr/GCE with 28Xf% Cr3+displays the best activity with a high response signal, a good sensitivity of 71.22 μA mM–1, a low detection limit of 0.1 μM (S/N = 3) and a fast response time (<3 s). Moreover, the reproducibility, selectivity and applicability of this electrochemical sensor are satisfactory evaluated.









Similar content being viewed by others
REFERENCES
Mikami, E., Goto, T., Ohno, T., Matsumoto, H., and Nishida, M., Simultaneous analysis of dehydroacetic acid, benzoic acid, sorbic acid and salicylic acid in cosmetic products by solid-phase extraction and high-performance liquid chromatography, J. Pharm. Biomed. Anal., 2002, vol. 28, p. 26.
Abdolmohammad-Zadeh, H., Kohansal, S., and Sadeghi, G.H., Nickel-aluminum layered double hydroxide as a nanosorbent for selective solid-phase extraction and spectrofluorometric determination of salicylic acid in pharmaceutical and biological samples, Talanta, 2011, vol. 84, p. 368.
Saha, U. and Baksi, K., Spectrophotometric determination of salicylic acid in pharmaceutical formulations using copper(II) acetate as a color developer, Int.J. Pharm., Anal., 2001, vol. 23, p. 95.
Zhang, W.D., Xu, B., Hong, Y.-X., Yu, Y.-X., Ye, J.-S., and Zhang, J.-Q., Electrochemical oxidation of salicylic acid at well-aligned multiwalled carbon nanotube electrode and its detection, J. Solid State Electrochem., 2010, vol. 14, p. 1713.
Zhihua, W., Xiaole, L., Bowan, W., Fangping, W., and Xiaoquan, L., Voltammetric Determination of salicylic acid by molecularly imprinted film modified electrodes, Int. J. Polym. Anal. Chem., 2012, vol. 17, p. 122.
Supalkova, V., Petrek, J., Havel, L., Krizkova, S., Petrlova, J., Adam, V., Potesil, D., Babula, P., Beklova, M., Horna, A., and Kizek, R., Electrochemical sensors for detection of acetylsalicylic acid, Sensors, 2006, vol. 6, p. 1483.
Ruiz-Medina, A., Fernàndez-de Córdova, M. L., Ortega-Barrales, P., and Molina-Díaz, A., Flow-through UV spectrophotometric sensor for determination of (acetyl)salicylic acid in pharmaceutical preparations, Int. J. Pharm., 2001, vol. 216, p. 95.
Kokot, Z. and Burda, K., Simultaneous determination of salicylic acid and acetylsalicylic acid in aspirin delayed-release tablet formulations by second derivative UV spectropohotometry, J. Pharm. Biomed. Anal., 1998, vol. 18, p. 871.
Marcelo, M.S., Marcello, G.T., and Ronei, J.P., Combining standard addition method and second-order advantage for direct determination of salicylate in undiluted human plasma by spectrofluorimetry, Talanta, 2006, vol. 68, p. 1707.
Kakkar, T. and Mayersohn, M., Simultaneous quantitative analysis of methyl salicylate, ethyl salicylate and salicylic acid from biological fluids using gas chromatography–mass spectrometry, J. Chromatogr. B, 1998, vol. 718, p. 69.
Kees, F., Jehnich, D., and Grobecker, H., Simultaneous determination of acetylsalicylic acid and salicylic acid in human plasma by high-performance liquid chromatography, J. Chromatogr. B, 1996, vol. 677, p. 172.
Jen, J.F., Tsai, Y.Y., and Yang, T.C., Microdialysis of salicylic acid from viscous emulsion samples prior to high-performance liquid chromatographic determination, J. Chromatogr. A, 2001, vol. 912, p. 39.
Petrek, J., Havel, L., Petrlova, J., Adam, V., Potesil, D., Babula, P., and Kizek, R., Analysis of salicylic acid in willow barks and branches by an electrochemical method, Russ. J. Plant. Physiol., 2007, vol. 54, p. 553.
Torriero, A.A.J., Luco, J.M., Sereno, L., and Raba, J., Voltammetric determination of salicylic acid in pharmaceuticals formulations of acetylsalicylic acid, Talanta, 2004, vol. 62, p. 247.
Rawlinson, S., McLister, A., Kanyong, P., and Davis, J., Rapid determination of salicylic acid at screen printed electrodes, Microchem. J., 2018, vol. 137, p. 71.
Park, J. and Eun, C., Electrochemical behavior and determination of salicylic acid at carbon-fiber electrodes, Electrochim. Acta, 2016, vol. 194, p. 346.
Wang, Z., Ai, F., Xu, Q., Yang, Q., Yu, J.H., Huang, W.H., and Zhao, Y.D., Electrocatalytic activity of salicylic acid on the platinum nanoparticles modified electrode by electrochemical deposition, Colloids Surf. B, 2010, vol. 76, p. 370.
Gualandi, I., Scavetta, E., Zappoli, S., and Tonelli, D., Electrocatalytic oxidation of salicylic acid by a cobalt hydrotalcite-like compound modified Pt electrode, Biosens. Bioelectron., 2011, vol. 26, p. 3200.
Alizadeh, T. and Nayeri, S., Electrocatalytic oxidation of salicylic acid at a carbon paste electrode impregnated with cerium-doped zirconium oxide nanoparticles as a new sensing approach for salicylic acid determination, J. Solid State Electrochem., 2018, vol. 22, p. 1.
Doulache, M., Saidat, B., and Trari, M., Electrocatalytic performance of cobalt microparticles film-modified platinum disk electrode for amperometric detection of ascorbic acid, J. Anal. Chem., 2017, vol. 72, p. 333.
Wei, Y., Wanga, A., and Liu, Y., Development of a glassy carbon electrode modified with graphene/Au nanoparticles for determination of acetaminophen in pharmaceutical preparation, Russ. J. Electrochem., 2018, vol. 54, p. 1141.
Babu, K.J., Senthilkumar, N., Kim, A.R., and Kumar, G.G., Three-dimensional dendrite Cu–Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection, Sens. Actuators B, 2017, vol. 241, p. 541.
Sanghavi, B.J., Kalambate, P.K., Karna, S.P., and Srivastava, A.K., Voltammetric determination of sumatriptan based on a graphene/gold nanoparticles/Nafion composite modified glassy carbon electrode, Talanta, 2014, vol. 120, p. 1.
Tadayon, F., Vahed, S., and Bagheri, H., Au–Pd/reduced graphene oxide composite as a new sensing layer for electrochemical determination of ascorbic acid, acetaminophen and tyrosine, Mater. Sci. Eng. C, 2016, vol. 68, p. 805.
Safaei, M., Beitollahib, H., and Shishehborea, M.R., Simultaneous determination of epinephrine and folic acid using the Fe3O4@SiO2/GR Nanocomposite Modified Graphite, Russ. J. Electrochem., 2018, vol. 54, p. 851.
Zhao, C., Li, M., and Jiao, K., Determination of formaldehyde by staircase voltammetry based on its electrocatalytic oxidation at a nickel electrode, J. Anal. Chem., 2006, vol. 61, p. 1204.
Majdi, S., Jabbari, A., and Heli, H., Electrocatalytic oxidation of some amino acids on a nickel-curcumin complex modified glassy carbon electrode, J. Solid State Electrochem., 2007, vol. 11, p. 601.
Safavi, A., Maleki, N., and Farjami, E., Fabrication of a glucose sensor based on a novel nanocomposite electrode, Biosens. Bioelectron., 2009, vol. 24, p. 1655.
Roushani, M., Shamsipur, M., and Pourmortazavi, S.M., Amperometric detection of glycine, L-Serine, and L‑Alanine using glassy carbon electrode modified by NiO nanoparticles, J. Appl. Electrochem., 2012, vol. 42, p. 1005.
Jafarian, M., Forouzandeh, F., Danaee, I., and Gobal, F., Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode, J. Solid State Electrochem., 2009, vol. 13, p. 1171.
Danaee, I., Jafarian, M., Mirzapoor, A., Gobal, F., and Mahjani, M.G., Electrooxidation of methanol on NiMn alloy modified graphite electrode, Electrochim. Acta, 2010, vol. 55, p. 2093.
Elahi, M.Y., Heli, H., Bathaie, S.Z., and Mousavi, M.F., Electrocatalytic oxidation of glucose at a Ni-curcumin modified glassy carbon electrode, J. Solid State Electrochem., 2007, vol. 11, p. 273.
Gholivanda, M.B., Pashabadi, A., Azadbakht, A., and Menati, S., A nano-structured Ni(II)-ACDA modified gold nanoparticle self-assembled electrode for electrocatalytic oxidation and determination of tryptophan, Electrochim. Acta, 2011, vol. 56, p. 4022.
Zheng, L. and Song, J.F., Nickel(II)-baicalein complex modified multiwall carbon nanotube paste electrode and its electrocatalytic oxidation toward glycine, Anal. Biochem., 2009, vol. 391, p. 56.
Zhong, D.C., Aranishi, K., Singh, A.K., Demirci, U.B., and Xu, Q., The synergistic effect of Rh–Ni catalysts on the highly-efficient dehydrogenation of aqueous hydrazine borne for chemical hydrogen, Chem. Commun., 2012, vol. 48, p. 1945.
Jiang, H.L. and Xu, Q., Porous metal-organic framewerks as platforms for function application, Mater. J. Chem., 2011, vol. 21, p. 3705.
Tao, B., Zhang, J., Hui, S., and Wan, L., An amperometric ethanol sensor based on a Pd–Ni/SiNWs electrode, Sens. Actuators B, 2009, vol. 142, p. 298.
Mattos, I.L., Melo, D., and Zagatto, E.A.G., Nickel-chromium electrode as a detector in flow-injection amperometry: determination of glycerol, Anal. Sci., 1999, vol. 15, p. 537.
Ojani, R., Raoof, J.B., and Norouzi, B., Performance of glucose electrooxidation on Ni–Co composition dispersed on the poly (isonicotinic acid)(SDS) film, J. Solid State Electrochem., 2011, vol. 15, p. 1139.
Yeo, I.H. and Johnson, D.C., Electrochemical response of small organic molecules at nickel-copper alloy electrodes, J. Electroanal. Chem., 2001, vol. 495, p. 110.
Connors, K.A., Amidon, G.L., and Kennon, L., Chemical Stability of Pharmaceuticals, New York: Wiley, 1979.
Marioli, J.M. and Sereno, L.E., The potentiodynamic behavior of nickel–chromium (80 : 20) alloy electrodes in 0.10 N sodium hydroxide, Electrochim. Acta, 1995, vol. 40, p. 983.
Marioli, J.M., Luo, P., and Kuwana, T., Nickel–chromium alloy electrode as a carbohydrate detector for liquid chromatography, Anal. Chim. Acta, 1993, vol. 282, p. 571.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, 2nd ed., John Wiley Press, 2001, p. 196.
Ghoreishi, S.M., Kashani, F.Z., Khoobi, A., and Enhessari, M., Fabrication of a nickel titanate nanoceramic modified electrode forelectrochemical studies and detection of salicylic acid, J. Mol. Liq., 2015, vol. 211, p. 970.
Kutner, W., Wang, J., Lher, M., and Buck, R.P., Analytical aspects of chemically modified electrodes: classification, critical evaluation and recommendations, Pure Appl. Chem., 1998, vol. 70, p. 1301.
Park, J. and Eun, C., Electrochemical behavior and determination of salicylic acid at carbon-fiber electrodes, Electrochim. Acta, 2016, vol. 194, p. 346.
ACKNOWLEDGMENTS
The authors would like to thank Dr M. Izeroukkene for his assistance in the SEM characterization.
Funding
The work is supported financially by the faculty of Chemistry (Algiers).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare have no conflict of interest financial, personal or other relationships with other people, laboratories or organizations.
Rights and permissions
About this article
Cite this article
Merzak Doulache, Mohamed Trari Electrocatalytic Determination of Salicylic Acid on Ni–Cr Alloy Modified Glassy Carbon Electrode. Russ J Electrochem 56, 615–625 (2020). https://doi.org/10.1134/S1023193520080042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520080042