Abstract
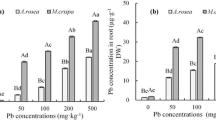
Mentha aquatica L., Eryngium caucasicum Trautv., and Froriepia subpinnata Ledeb are native plants in Iran. Lead (Pb) is one of the most dangerous heavy metals that damages to plant growth process. In the present study, we investigated the biochemical response of M. aquatica, E. caucasicum and F. subpinnata to Pb stress in the three separate pot experiments. Experimental treatments were different concentrations of Pb including 0, 125, 250, 375, and 500 mg/kg of Pb in the soil. Chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoid, phenol, flavonoid, DPPH, and Pb concentration in the shoot was measured. The result showed that Pb stress significantly affected photosynthesis pigments. The minimum Chl a, Chl b, and carotenoid content was produced at 500 mg/kg of Pb in the soil in all three plants. Total phenol and flavonoid compounds and antioxidant capacity of plants increased with enhancement of Pb concentration in soil. M. aquatica produced the maximum total phenol and total flavonoid compounds, and antioxidant activity compared to E. caucasicum and F. subpinnata. Enhancement of antioxidant compounds production due to the Pb stress could be a defense mechanism to tolerate Pb stress. The concentration of Pb in the shoot of all three plants was increased linearly with the enhancement in the concentration of Pb in soil. M. aquatica had the lowest concentration of Pb in the shoot. Also, M. aquatica was more resistant to Pb stress than the other two plants due to the lower effect of Pb stress on photosynthetic pigments and higher antioxidant compounds content.







Similar content being viewed by others
REFERENCES
Islam, E., Liu, D., and Li, T.Q., Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi, J. Hazard. Mater., 2008, vol. 154, p. 914. https://doi.org/10.1016/j.jhazmat.2007.10.121
Ashraf, U., Kanu, A.S., Mo, Z., Hussain, S., Anjum, S.A., Khan, I., Abbas, R.N., and Tang, X., Lead toxicity in rice: effects, mechanisms, and mitigation strategies – A mini review, Environ. Sci. Pollut. Res., 2015, vol. 22, p. 18318. https://doi.org/10.1007/s11356-015-5463-x
Anjum, N.A., Ahmad, I., Mahmood, I., Pacheco, M., Duarte, A.C., Pereira, E., Umar, S., Ahmad, A., Khan, N., Iqbal, M., and Prasad, M.N.V., Modulation of glutathione and its related enzymes in plants' responses to toxic metals and metalloids – A review, Environ. Exp. Bot., 2012, vol. 75, p. 307. https://doi.org/10.1016/j.envexpbot.2011.07.002
Nas F.S. and Ali, M., The effect of lead on plants in terms of growing and biochemical parameters: a review, MOJ Eco. Environ. Sci., 2018, vol. 3, p. 265. https://doi.org/10.15406/mojes.2018.03.00098
Rucińska-Sobkowiak R., Water relations in plants subjected to heavy metal stresses, Acta. Physiol. Plant., 2016, vol. 38, p. 257. https://doi.org/10.1007/s11738-016-2277-5
Tohidi Moghadam, H.R., Donath, T.W., Ghooshchi, F.M., and Sohrabi, M., Investigating the probable consequences of super absorbent polymer and mycorrhizal fungi to reduce detrimental effects of Pb on wheat (Triticum aestivum L.), Agron. Res., 2018, vol. 16, p. 286. https://doi.org/10.15159/AR.18.009
Kumar, G.H. and Kumari, J.P., Heavy metal lead influative toxicity and its assessment in phytoremediating plants – A review., Water, Air, Soil Pollut., 2015, vol. 226, 324. https://doi.org/10.1007/s11270-015-2547-7
Emamverdian, A., Ding, Y., Mokhberdoran, F., and Xie, Y., Heavy metal stress and some mechanisms of plant defense response, Sci. World J., 2015, vol. 18. https://doi.org/10.1155/2015/756120
Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M., and Zheng, B., Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress, Molecules, 2019, vol. 24, p. 2452. https://doi.org/10.3390/molecules24132452
Venditti A., Frezza, C., Celona, D., Sciubba, F., Foddai, S., Delfini, M., Serafini, M., and Bianco, A., Phytochemical comparison with quantitative analysis between two flower phenotypes of Mentha aquatica L.: pink-violet and white, AIMS Molecular Sci., 2017, vol. 4, p. 288. https://doi.org/10.3934/molsci.2017.3.288
Behmanesh, E., Delavar, M.A., Kamalinejad, M., Khafri, S., Shirafkan, H., and Mozaffarpur, S.A., Effect of eryngo (Eryngium caucasicum Trautv) on primary dysmenorrhea: A randomized, double-blind, placebo-controlled study, Taiwan J. Obstet. Gynecol., 2019, vol. 58, p. 227. https://doi.org/10.1016/j.tjog.2019.01.011
Mehdiyeva N.P., Alizade, V.M., Zambrana, N.Y.P., and Bussmann, R.W., Froriepia subpinnata (Ldb.) Bail Apiaceae. In: Bussmann R. (eds.) Ethnobotany of the Caucasus. European Ethnobotany. Springer, Cham., 2017, p. 313. https://doi.org/10.1007/978-3-319-49412-8_49
Morteza-Semnani, K., Saeedi, M., and Akbarzadeh, M., The essential oil composition of Mentha aquatica L., J. Essent. Oil Bear. Plants, 2006, vol. 9, p. 283. https://doi.org/10.1080/0972060X.2006.10643505
Ahmad, M., Usman, A.R.A., Al-Faraj, A.S., Ahmad, M., Sallam, A., and Al-Wabel, M.I., Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants, 2018, Chemosphere, vol. 194, p. 327. https://doi.org/10.1016/j.chemosphere.2017.11.156
Chandra, R. and Hoduck, K., Phytoremediation and physiological effects of mixed heavy metals on poplar hybrids, Heavy Metals, Saleh H.E.M., Aglan R. (eds.), London: IntechOpen; 2018, p. 412. https://doi.org/10.5772/intechopen.76348
Porra, R.J., The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b, Photosynth Res., 2002, vol. 73, p. 149. https://doi.org/10.1023/A:1020470224740
Farhoosh R. and Moosavi, S.M.R., Determination of carbonyl value in rancid oils: A critical reconsideration, J. Food Lipids, 2006, vol. 13, p. 298. https://doi.org/10.1111/j.1745-4522.2006.00053.x
Ordonez, A.A.L., Gomez, J.D., Vattuone, M.A., and lsla, M.I., Antioxidant activities of Sechium edule (Jacq.) Swartz extracts, Food Chem., 2006, vol. 97, p. 452. https://doi.org/10.1016/j.foodchem.2005.05.024
Nabavi, S.M., Ebrahimzadeh, M.A., Nabavi, S.F., Fazelian, M., and Eslami, B., In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran, Pharmacogn. Mag., 2009, vol. 5, p. 122.
Sanchez-Moreno, C., Larrauri, J.A., and Saura-Calixto, F., Free radical scavenging capacity of selected red, rose and white wines, J. Agric. Food Chem., 1999, vol. 79, p. 1301. https://doi.org/10.1016/S0963-9969(99)00097-6
Woodis, Jr. T.C., Hunter, G.B., and Johnson, F.J., Statistical studies of matrix effects on the determination of cadmium and lead in fertilizer and material and plant tissue by flame atomic absorption spectrophotometry, Anal. Chim. Acta, 1977, vol. 90, p. 127. https://doi.org/10.1016/S0003-2670(01)82301-1
Mani, D., Kumar, C., and Patel, N.K., Hyperaccumulator oilcake manure as an alternative for chelate-induced phytoremediation of heavy metals contaminated alluvial soils., Int. J. Phytorem., 2015, vol. 17, p. 256. https://doi.org/10.1080/15226514.2014.883497
Dogan, M., Karatas, M., and Aasim, M., Cadmium and lead bioaccumulation potentials of an aquatic macrophyte Ceratophyllum demersum L.: A laboratory study, Ecotoxicol. Environ. Saf., 2018, vol. 148, p. 431. https://doi.org/10.1016/j.ecoenv.2017.10.058
Cenkci, S., Cigerci, I.H., Yildiz, M., Ozay, C., Bozdag, A., and Terzi, H., Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L., Environ. Exp. Bot., 2010, vol. 67, p. 467. https://doi.org/10.1016/j.envexpbot.2009.10.001
Ferreyroa, G.V., Lagorio, M.G., Trinelli, M.A., Lavado, R.S., and Molina, F.V., Lead effects on Brassica napus photosynthetic organs, Ecotoxicol. Environ. Saf., 2017, vol. 140, p. 123. https://doi.org/10.1016/j.ecoenv.2017.02.031
Sharma, P. and Dubey, R.S., Lead toxicity in plants, Braz. J. Plant Physiol., 2005. vol. 17, p. 35. https://doi.org/10.1016/j.bcab.2015.03.004
Majumdar R., Barchi, B., Turlapati, S.A., Gagne, M., Minocha, R., Long, S., and Minocha, S.C., Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level, Front. Plant Sci., 2016, vol. 7, p. 78. https://doi.org/10.3389/fpls.2016.00078
Haider, S., Kanwal, S., Uddin, F., and Azmat, R., Phytotoxicity of Pb II. Changes in chlorophyll absorption spectrum due to toxic metal Pb stress on Phaseolus mungo and Lens culinaris, Pak. J. Biol. Sci., 2006, vol. 9, p. 2062. https://doi.org/10.3923/pjbs.2006.2062.2068
Jayasri, M.A. and Suthindhiran, K., Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: its potential role in phytoremediation, Appl. Water Sci., 2017, vol. 7, p. 1247. https://doi.org/10.1007/s13201-015-0376-x
Singh, S., Parihar, P., Singh, R., Singh, V.P., and Prasad, S.M., Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics, Front. Plant. Sci., 2015, vol. 6, p. 1. https://doi.org/10.3389/fpls.2015.01143
Frank, H. and Brudvig, G.W., Redox functions of carotenoids in photosynthesis, Biochem., 2004, vol. 43, p. 8607. https://doi.org/10.1021/bi0492096
Yang, Y., Zhang, L., Huang, X., Zhou, Y., Quan, Q., Li, Y., and Zhu, X., Response of photosynthesis to different concentrations of heavy metals in Davidia involucrate, PLoS One, 2020, vol. 15, p. 6. https://doi.org/10.1371/journal.pone.0228563
Khan, W., Subhan, S., Shams, D.F., Afridi, S.G., Ullah, R., Shahat, A.A., and Alqahtani, A.S., Antioxidant potential, phytochemicals composition, and metal contents of Datura alba., Bio. Med. Res. Inter., 2019, p. 8. https://doi.org/10.1155/2019/2403718
Kýsa, D., Elmastas, M., Ozturk, L., and Kayir, O., Responses of the phenolic compounds of Zea mays under heavy metal stress, Appl. Biol. Chem., 2016, vol. 59, p. 813. https://doi.org/10.1007/s13765-016-0229-9
Bystrická, J., Árvay, J., Musilová, J., Vollmannová, A., Tóth, T., and Lenková, M., The investigation of sensitivity of different types of onion to heavy metal intake from contaminated soil, Int. J. Environ. Res., 2016, vol. 10, p. 427. https://doi.org/10.22059/ijer.2016.58762
Tosun, M., Ercisli, S., Sengul, M., Ozer, H., Polat, T., and Ozturk, E., Antioxidant properties and total phenolic content of eight Salvia species from Turkey, Biol. Res., 2009, vol. 41, p. 175. https://doi.org/10.4067/S0716-97602009000200005
Sellal, A., Belattar, R., and Bouzidi, A., Heavy metals chelating ability and antioxidant activity of Phragmites australis stems extracts, Ecol. Eng., 2019, vol. 20, p. 116. https://doi.org/10.12911/22998993/96276
Khalid, N., Noman, A., Aqeel, M., Masood, A., and Tufail, A., Phytoremediation potential of Xanthium strumarium for heavy metals contaminated soils at roadsides, Int. J. Environ. Sci. Technol., 2018, vol. 16, p. 2091. https://doi.org/10.1007/s13762-018-1825-5
Song, F.L., Gan, R.Y., Zhang, Y., Xiao, Q., Kuang, L., and Li, H.B., Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants, Int. J. Mol. Sci, 2010, vol. 11, p. 2362. https://doi.org/10.3390/ijms11062362
Zhang, Q., Zhang, M., Ding, Y., Zhou, P., and Fanga, Y., Composition of photosynthetic pigments and photosynthetic characteristics in green and yellow sectors of the variegated Aucuba japonica ‘Variegata’ leaves, Flora, 2018, vol. 240, p. 25.
ACKNOWLEDGMENTS
The authors wish to acknowledge Sari Agricultural Sciences and Natural Resources University (Sari, Iran) for financial support of this study.
Funding
Funding was provided by Sari Agricultural Sciences and Natural Resources University.
Author information
Authors and Affiliations
Contributions
Roghayeh Hasanpour conducted the experiment, gathered and analyzed data, and contributed to the writing and discussion of the manuscript. Faezeh Zaefarian was involved in the conception and design of the experiment, conducted the experiment, and contributed to the discussion of the manuscript. Mohammad Rezvani revised the manuscript and contributed to the discussion of the manuscript. Bahi Jalili involved in the conception and design of the experiment, and revision of the manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants as objects of research.
Rights and permissions
About this article
Cite this article
Hasanpour, R., Zaefarian, F., Rezvani, M. et al. Physiological Response of Mentha aquatica L., Eryngium caucasicum (Trautv.), and Froriepia subpinnata (Ledeb.) to Lead Stress. Russ J Plant Physiol 69, 95 (2022). https://doi.org/10.1134/S1021443722050089
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722050089