Abstract
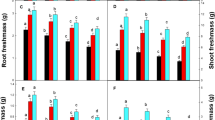
The effects of increase copper concentrations in medium (10–150 μM CuSO4) on growth and viability of the roots of two-week-old soybean seedlings (Glycine max L., cv. Dorintsa) were studied. Copper excess suppressed biomass accumulation and linear plant growth; copper affected root growth much stronger than shoot growth. The presence of 10 μM CuSO4 in medium suppressed accumulation of plant biomass by 40% and the root length by 70%; in the presence of 25 μM CuSO4, these indices were equal to 80 and 90%, respectively. In the presence of 50 μM CuSO4, roots ceased to grow but biomass and shoot length still increased slightly. 150 μM CuSO4 was lethal for plants. The earliest sign of excessive copper toxicity was the accumulation of MDA, indicating activation of membrane lipid peroxidation. A significant increase in MDA content was observed at plant incubation in medium with 10 μM CuSO4 for 1 h; in this case, the content of copper in the roots increased from 36 ±1.8 (in control) to 48 ± 2.4 μg/g dry wt. The number of dead cells (permeable for the dye Evans Blue) was doubled in the presence of 200 μg/g dry wt within the root; this occurred in 72 h of growth in medium with 10 μM CuSO4, in 6 h at 25 μM CuSO4, in 3 h at 50 μM CuSO4, and 1 h at 150 μM CuSO4. Toxicity of copper excess was manifested stronger in dividing and elongation cells of the root apex (root meristem and the zone of elongation) than in more basal root regions. Copper excess resulted in the formation of breaks in the surface cell layers of the root tips and affect root morphology. When plant grew in medium with 10 μM CuSO4, a distance of lateral root formation zone from the root tip decreased markedly, and spherical swellings were formed on the tips of lateral roots. The higher copper concentrations (50 and 150 μM) suppressed completely the development of lateral roots.
Similar content being viewed by others
Abbreviations
- HM:
-
heavy metal
- POL:
-
peroxidation of lipids
- TBA:
-
thiobarbituric acid
References
Fernandes, J.C. and Henriques, F.S., Biochemical, Physiological, and Structural Effects of Excess Copper in Plants, Bot. Rev., 1991, vol. 57, pp. 246–273.
Maksymiec, W., Effect of Copper on Cellular Processes in Higher Plants, Photosynthetica, 1997, vol. 34, pp. 360–368.
Yruela, I., Copper in Plants: Acquisition, Transport and Interactions, Funct. Plant Biol., 2009, vol. 36, pp. 409–430.
Hall, J.L., Cellular Mechanisms for Heavy Metal Detoxification and Tolerance, J. Exp. Bot., 2002, vol. 53, pp. 1–11.
De Vos, C.H.R., Schat, H., Vooijs, R., and Ernst, W.A.O., Copper Induced Damage to the Permeability Barrier in Roots of Silene cucubalus, J. Plant Physiol., 1989, vol. 135, pp. 164–169.
MacFarlaine, G.R. and Burchett, M.D., Toxicity, Growth and Accumulation Relationships of Copper, Lead, Zink in the Grey Mangrove Avicenia marina (Forsk.) Vierh., Marine Environ. Res., 2002, vol. 54, pp. 65–84.
Kopittke, P.M. and Menzies, N.W., Effect of Cu Toxicity on Growth of Cowpea (Vigna unguiculata), Plant Soil, 2006, vol. 279, pp. 287–296.
Mourato, M.P., Martins, L.L., and Campos-Andrada, M.P., Physiological Responses of Lupinus luteus to Different Copper Concentrations, Biol. Plant., 2009, vol. 53, pp. 105–111.
Yamamoto, Y., Kobayashi, Y., and Matsumoto, H., Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, but Not the Primary Cause of Elongation Inhibition in Pea Roots, Plant Physiol., 2001, vol. 125, pp. 199–208.
Kopittke, P.M., Blamey, F.P.C., and Menzies, N.W., Toxicities of Soluble Al, Cu, and La Include Ruptures to Rhizodermal and Root Cortical Cells of Cowpea, Plant Soil, 2008, vol. 303, pp. 217–227.
Kopittke, P.M., Blamey, F.P.C., Asher, C.J., and Menzies, N.W., Trace Metal Phytotoxicity in Solution Culture: A Review, J. Exp. Bot., 2010, vol. 61, pp. 945–954.
Pintro, J.C. and Taylor, G.J., Effect of Aluminium Toxicity on Wheat Plants Cultivated under Conditions of Varying Ionic Strength, J. Plant Nutr., 2004, vol. 27, pp. 907–919.
Zhou, Z.S., Huang, S.Q., Guo, K., Mehta, S.K., Zhang, P.C., and Yang, Z.M., Metabolic Adaptations to Mercury-Induced Oxidative Stress in Roots of Medicago sativa L., J. Inorg. Biochem., 2007, vol. 101, pp. 1–9.
Heath, R.I. and Packer, I., Photoperoxidation in Isolated Chloroplast: 1. Kinetics and Stoichiometry of Fatty Acid Peroxidation, Arch. Biochem. Biophys., 1968, vol. 125, pp. 180–198.
Bernal, M., Cases, R., Picorel, R., and Yruela, I., Foliar and Root Cu Supply Affect Differently Fe- and Zn-Uptake and Photosynthetic Activity in Soybean Plants, Environ. Exp. Bot., 2007, vol. 60, pp. 145–150.
Lin, S.L. and Wu, L., Effect of Copper Concentration on Mineral Nutrient Uptake and Copper Accumulation in Protein of Copper Tolerant and Nontolerant Lotus purshianus L., Ecotoxicol. Environ. Saf., 1994, vol. 29, pp. 214–228.
Kopittke, P.M., Asher, C.J., Blamey, F.P.C., and Menzies, N.W., Toxic Effect of Cu2+ on Growth, Nutrition, Root Morphology, and Distribution of Cu in Roots of Sabi Grass, Sci. Total Environ., 2009, vol. 407, pp. 4616–4621.
Lequeux, H., Hermans, C., Lutts, S., and Verbruggen, N., Response to Copper Excess in Arabidopsis thaliana: Impact on the Root System Architecture, Hormone Distribution, Lignin Accumulation and Mineral Profile, Plant Physiol. Biochem., 2010, vol. 48, pp. 673–682.
Madejon, P., Ramires-Benitez, J.E., Corrales, I., Barcelo, J., and Poschenrieder, C., Copper-Induced Oxidative Damage and Enhanced Antioxidant Defenses in the Root Apex of Maize Cultivars Differing in Cu Tolerance, Environ. Exp. Bot., 2009, vol. 67, pp. 415–420.
Sancenon, V., Puig, S., Mateu-Andres, I., Dorcey, E., Thiele, D.J., and Penarrubia, L., The Arabidopsis Copper Transporter COPT1 Functions in Root Elongation and Pollen Development, J. Biol. Chem., 2004, vol. 279, pp. 15348–15355.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.L. Kulikova, N.A. Kuznetsova, V.P. Kholodova, 2011, published in Fiziologiya Rastenii, 2011, Vol. 58, No. 5, pp. 719–727.
Rights and permissions
About this article
Cite this article
Kulikova, A.L., Kuznetsova, N.A. & Kholodova, V.P. Effect of copper excess in environment on soybean root viability and morphology. Russ J Plant Physiol 58, 836–843 (2011). https://doi.org/10.1134/S102144371105013X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102144371105013X