Abstract
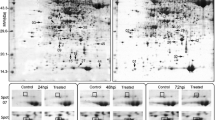
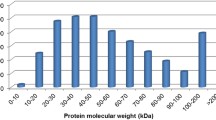
Wheat powdery mildew is caused by Blumeria graminis f. sp. tritici (Bgt). Pm21 is an effective broad-spectrum powdery mildew resistance gene, which shows a considerable promise in wheat breeding. We report here a proteomic approach to investigate the resistance response proteins after fungal infection and emphasize the resistance changes induced by Pm21. Two wheat (Triticum aestivum L.) near-isogenic lines (NILs), recurrent parent ‘Bainong,’ which is susceptible to powdery mildew, and its near-isogenic line ‘W2132’ carrying resistance gene Pm21) were used to investigate some changes in their proteomes after being infected. Proteins were extracted from the leaves sampled in 48 h after inoculation, separated by two-dimensional electrophoresis, and stained with Coomassie brilliant blue. Among these proteins, a total of 56 spots differentially expressed after Bgt infection were detected. Sixteen proteins, identified by MALDI-TOF-MS, exhibited more than a 1.5-fold increase upon fungal infection. Unfortunately, three spots were not identified successfully. The predicted functions of identified proteins were related to energy metabolism and defensive responses; they were involved in many physiological resistance responses, including enhancing energy metabolism, proteins synthesis and stabilization, antioxidant reactions, cell-wall reinforcement, and lignification. Interestingly that the expression of two proteins related to the cell-wall reinforcement was enhanced in the resistant line and one protein related to photosynthesis was lost in a susceptible line. By transmission electronic microscopy, the corresponding physiological characteristics were also observed. These results provide us with the information to further reveal the resistance mechanism of Pm21 action and comprehensively investigate the physiological response to powdery mildew at the protein level.
Similar content being viewed by others
Abbreviations
- Bgt:
-
Blumeria graminis f. sp. tritici
- IEF:
-
isoelectric focusing
- NIL:
-
near-isogenic line
- MALDI-TOF-MS:
-
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- TEM:
-
transmission electron microscopy
- 2-DE:
-
two-dimensional electrophoresis
References
Eichmann, R. and Hückelhoven, R., Accommodation of Powdery Mildew Fungi in Intact Plant Cells, J. Plant Physiol., 2008, vol. 165, pp. 5–18.
Babosha, A.V., Ryabchenko, A.S., and Avetisyan, T.V., Effect of Exogenous Cytokinins on Dynamics of Development and Differentiation of Infectious Structures of the Pathogen of Wheat Powdery Mildew, Cell Tissue Biol., 2009, vol. 3, pp. 387–396.
Deng, Z.P., Zhang, X., Tang, W.Q., Oses-Prieto, J.A., Suzuki, N., Gendron, J.M., Chen, H.J., Guan, S.H., Chalkley, R.J., and Peterman, T.K., A Proteomics Study of Brassinosteroid Response in Arabidopsis, Mol. Cell. Proteomics, 2007, vol. 6, pp. 2058–2071.
Campo, S., Carrascal, M., Coca, M., Abián, J., and San Segundo, B., The Defense Response of Germinating Maize Embryos against Fungal Infection: A Proteomics Approach, Proteomics, 2004, vol. 4, pp. 383–396.
Rampitsch, C., Bykova, N.V., McCallum, B., Beimcik, E., and Ens, W., Analysis of the Wheat and Puccinia triticina (Leaf Rust) Proteomes during a Susceptible Host-Pathogen Interaction, Proteomics, 2006, vol. 6, pp. 1897–1907.
Shevchenko, A., Sunyaev, S., and Loboda, A., Charting the Proteomes of Organisms with Unsequenced Genomes by MALDI-Quadrupole Time-of-Flight Mass Spectrometry and BLAST Homology Searching, Anal. Chem., 2001, vol. 73, pp. 1917–1926.
Zhou, R.H., Zhu, Z.D., Kong, X.Y., Huo, N.X., Tian, Q.Z., Li, P., Jin, C.Y., Dong, Y.C., and Jia, J.Z., Development of Wheat Near-Isogenic Lines for Powdery Mildew Resistance, Theor. Appl. Genet., 2005, vol. 110, pp. 640–648.
Huang, X.Q. and Hsam, S.L.K., and Zeller, F.J., Identification of Powdery Mildew Resistance Genes in Common Wheat (Triticum aestivum L.). IX. Cultivars, Landraces and Breeding Lines Grown in China, Plant Breed., 1997, vol. 116, pp. 233–238.
Musetti, R., Bruni, L., and Favali, M.A., Cytological Modifications in Maize Plants Infected by Barley Yellow Dwarf Virus and Maize Dwarf Mosaic Virus, Micron, 2002, vol. 33, pp. 681–686.
Liu, Z., Sun, Q., Ni, Z., Yang, T., and McIntosh, R.A., Development of SCAR Markers Linked to the Pm21 Gene Conferring Resistance to the Powdery Mildew in Common Wheat, Plant Breed., 1999, vol. 118, pp. 215–219.
Mittler, R., Oxidative Stress, Antioxidants and Stress Tolerance, Trends Plant Sci., 2002, vol. 7, pp. 405–410.
Tariq, M., Asad, J., Makoto, K., and Setsuko, K., Proteomic Analysis of Bacterial-Blight Defense Responsive Proteins in Rice Leaf Blades, Proteomics, 2006, vol. 6, pp. 6053–6065.
Parry, M.A.J., Andralojc, P.J., Khan, S., Lea, P.J., and Keys, A.J., Rubisco Activity: Effects of Drought Stress, Ann. Bot., 2002, vol. 89, pp. 833–839.
Soulages, J.L., Kim, K., Arrese, E.L., Walters, C., and Cushman, J.C., Conformation of a Group 2 Late Embryogenesis Abundant Protein from Soybean. Evidence of Poly (L-Proline)-Type Structure, Plant Physiol., 2003, vol. 131, pp. 963–975.
Hara, M., Fujinaga, M., and Kuboi, T., Radical Scavenging Activity and Oxidative Modification of Citrus Dehydrin, Plant Physiol. Biochem., 2004, vol. 42, pp. 657–662.
Caruso, A., Morabito, D., Delmatte, F., Kahlem, G., and Carpin, S., Dehydrin Induction during Drought and Osmotic Stress in Populus, Plant Physiol. Biochem., 2002, vol. 40, pp. 1033–1042.
Spreitzer, R.J., Role of the Small Subunit of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase, Arch. Biochem. Biophys., 2003, vol. 414, pp. 141–149.
Romero, A.D., Rivera, M.E., Cazorla, M.F., Codina, J.C., Fernández-Ortuño, D., Torés, J.A., and Pérez-Garcia, A., Comparative Histochemical Analyses of Oxidative Burst and Cell Wall Reinforcement in Compatible and Incompatible Melon-Powdery Mildew (Podosphaera fusca) Interactions, J. Plant Physiol., 2008, vol. 165, pp. 1895–1905.
Györgyey, J., Németh, K., Magyar, Z., Kelemen, Z., Alliotte, T., Inzé, D., and Dudits, D., Expression of a Novel-Type Small Proline-Rich Gene of Alfalfa Is Induced by 2,4-Dichlorophenoxiacetic Acid in Dedifferentiated Callus Cells, Plant Mol. Biol., 1997, vol. 34, pp. 593–602.
Bradley, D.J., Kjellbom, P., and Lamb, C.J., Elicitor- and Wound-Induced Oxidative Cross-Linking of a Proline-Rich Plant Cell Wall Protein: A Novel, Rapid Defence Response, Cell, 1992, vol. 70, pp. 21–30.
Brisson, L.F., Tenhaken, R., and Lamb, C., Function of Oxidative Cross-Linking of Cell Wall Structural Proteins in Plant Disease Resistance, Plant Cell, 1994, vol. 6, pp. 1703–1712.
Schwarz, G., Michalek, W., Jahoor, A., and Mohler, V., Direct Selection of Expressed Sequences on a YAC Clone Revealed Proline-Rich-Like Genes and BARE-1 Sequences Physically Linked to the Complex Mla Powdery Mildew Resistance Locus of Barley (Hordeum vulgare L.), Plant Sci., 2002, vol. 163, pp. 307–311.
Yu, G.H., and An, G., Regulatory Roles of Benzyladenine and Sucrose during Wound Response of the Ribosomal Protein Gene, rpL34, Plant Cell Environ., 2000, vol. 23, pp. 1363–1371.
Kim, K.Y., Park, S.W., Chung, Y.S., Chung, C.H., Kim, J.I., and Lee, J.H., Molecular Cloning of Low-Temperature-Inducible Ribosomal Proteins from Soybean, J. Exp. Bot., 2004, vol. 55, pp. 1153–1155.
Zhang, S.Y., Scott, J.M., and Haldenwang, W.G., Loss of Ribosomal Protein L11 Blocks Stress Activation of the Bacillus subtilis Transcription Factor σ B, J. Bacteriol., 2001, vol. 183, pp. 2316–2321.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Fiziologiya Rastenii, 2011, Vol. 58, No. 4, pp. 590–598.
This text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Li, Q., Chen, X.M., Li, D. et al. Differences in protein expression and ultrastructure between two wheat near-isogenic lines affected by powdery mildew. Russ J Plant Physiol 58, 686–695 (2011). https://doi.org/10.1134/S102144371104008X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102144371104008X