Abstract
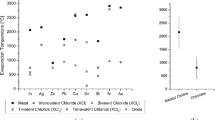
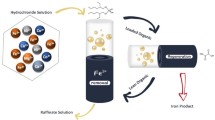
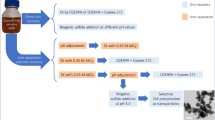
Calculations and experimental analysis of phosphate ores contaminated with Fe(III) compounds showed that advanced processing of these ores in a scheme with recycling of Fe(III) compounds is possible. The selective precipitation of \({\text{PO}}_{4}^{{3 - }}\) ions from a nitric acid extract with Fe(III) ions in the form of a FePO4 precipitate, the subsequent alkaline extraction of \({\text{PO}}_{4}^{{3 - }}\) ions from FePO4, and the dissolution of the formed Fe(OH)3 precipitate in HNO3 make it possible to obtain soluble phosphates and to recycle Fe(III) ions for the precipitation of \({\text{PO}}_{4}^{{3 - }}\) from the nitric acid extract. The residual P(V) content in the Fe(OH)3 precipitate under the conditions of the recycling of Fe(III) ions is 0.80 wt %, which corresponds to a degree of \({\text{PO}}_{4}^{{3 - }}\) extraction from FePO4 of 97.1%. The results of the theoretical calculations agree with the obtained experimental data. The proposed original solutions make it possible to process more phosphate ore contaminated with Fe(III) compounds with smaller amounts of Fe(III) compounds, including those contained in the ore.








Similar content being viewed by others
REFERENCES
State Report “On the State and Use of Mineral Resources of the Russian Federation in 2016 and 2017,” Ministry of Natural Resources and Environment of the Russian Federation, Moscow, 2018.
Executive Order of the Government of the Russian Federation no. 2914-r of December 22, 2018, “On the Strategy of the Development of Mineral Resources of the Russian Federation to 2035,” Moscow, 2018.
Babkin, V.V., Phosphorus Fertilizers of Russia, Moscow: Magnus, 1996.
Kijkowska, R., Pawlowska-Kozinska, D., Kowalski, Z., Jodko, M., and Wzorek, Z., Wet-process phosphoric acid obtained from Kola apatite: Purification from sulphates, fluorine, and metals, Sep. Purif. Technol., 2002, vol. 28, no. 3, pp. 197–205. https://doi.org/10.1016/S1383-5866(02)00048-5
Technology of Phosphorus and Complex Fertilizers, Evenchik, S.D. and Brodskii, A.A., Eds., Moscow: Khimiya, 1987.
Karmyshov, V.F., Chemical Processing of Phosphorites, Moscow: Khimiya, 1983.
Bingqi Wang., Lin Yang., Tong Luo., and Jianxin Cao., Study on the kinetics of hydration transformation from hemihydrate phosphogypsum to dihydrate phosphogypsum in simulated wet process phosphoric acid, ACS Omega, 2021, no. 6, pp. 7342−7350. https://doi.org/10.1021/acsomega.0c05432
Soussi-Baatout, A., Brahim, Kh., Khattech, I., Kamoun, L., and Jemal, M., Thermochemical and kinetic investigations of the phosphoric attack of Tunisian phosphate ore, J. Therm. Anal. Calorim., 2018, no. 131, pp. 3121–3132. https://doi.org/10.1007/s10973-017-6825-z
Technology of Phosphorus and Complex Fertilizers, Evenchik, S.D. and Brodskii, A.A,, Eds., Moscow: Khimiya, 1987.
Petropavlovskii, I.A., Pochitalkina, I.A., and Ryashko, A.I., Graphic study of the dihydrate–hemihydrate process for the synthesis of phosphoric acid according to the diagram of the CaO–P2O5–SO3–H2O system, Theor. Found. Chem. Eng., 2019, vol. 53, no. 3, pp. 364–369.
Angelov, A.I., et al., Production of enriched superphosphate from low-grade phosphate raw materials of the Yegor’evskoe deposit, Khim. Prom-st. Segodnya, 2006, no. 1, pp. 13–21.
Pérez-López, R., Macias, F., Cánovas, C.R., Sarmiento, A.M., and Pérez-Moreno, S.M., Pollutant flows from a phosphogypsum disposal area to an estuarine environment: An insight from geochemical signatures, Sci. Total Environ., 2016, vol. 553, pp. 42−51. https://doi.org/10.1016/j.scitotenv.2016.02.070
Hang Ma., Xiao Feng., and Bo Zeng., Self-anticorrosion for the combustion tower of heat recovered thermal process phosphoric acid production, Process Saf. Environ. Prot., 2018, vol. 118, pp. 330–347. https://doi.org/10.1016/j.psep.2018.07.008
Dobrydnev, S.V., Bogach, V.V., Pochitalkina, I.A., and Beskov, V.S., Ionometric study of the acid decomposition of phosphate minerals, Theor. Found. Chem. Eng., 2001, vol. 35, no. 3, pp. 292−297.
Dobrydnev, S.V., Pochitalkina, I.A., Bogach, V.V., and Beskov, V.S., Potentiometric (acidimetric) study of the fluoroapatite concentrate decomposition reaction with nitric acid, Chem. Ind., 2000, vol. 54, nos. 7−8, pp. 319−323.
Iretskaya, S.N., et al., Production of controlled-release fertilizers from carbonate-containing phosphorites and intermediate products of nitric acid processing of phosphate raw materials, Zh. Prikl. Khim., 1993, vol. 66, no. 9, pp. 1921−1926.
Dmitrevskii, B.A., et al., Solubility in the system Ca2+, NH4 +, PO4 3−, NO3 −, Zh. Prikl. Khim., 1988, no. 3, pp. 623−625.
Pochitalkina, I.A., et al., Behavior of impurities in phosphorite from the Polpinskoe deposit in the process of acid extraction, Zh. Neorg. Khim., 2018, vol. 63, no. 5, pp. 1−4.
Petropavlovskii, I.A. and Bespalov, A.V., Kinetics of acid dissolution of highly reactive natural phosphates, Teor. Osn. Khim. Tekhnol., 1988, vol. 22, no. 3, pp. 397−400.
Concise Handbook of Chemistry, Pilipenko, A.T., Ed., Kiev: Naukova Dumka, 1987.
GOST 20851.2-75, Mineral Fertilizers: Phosphate Determination Methods, USSR State Standard. Official Edition. Effective as of January 1, 1976. Moscow: Izd. Standartov, 1997, p. 17.
Vinnik, M.M., Erbanova, L.N., Zaitsev, P.M., et al., Methods of Analyzing Phosphate Raw Materials, Phosphate and Complex Fertilizers, and Feed Phosphates, Moscow: Khimiya, 1975.
Analytical Chemistry: Chemical Methods of Analysis, Petrukhin, O.M., Ed., Moscow: Khimiya, 1992.
Ekspert-001 Liquid Analyzers: Operation Manual and Verification Procedure, Moscow: Ekoniks-Ekspert, 2007.
Angelov, A.I., et al., Processing of regional phosphorites into qualified phosphorus fertilizers, Khim. Prom-st., 1996, no. 11, pp. 18−26.
Angelov, A.I., et al., Outlooks of the involvement of low-grade phosphate raw materials in production, in Proceedings of the Samoilov Research Institute for Fertilizers and Insectofungicides (NIUIF): To the 85 Anniversary of the NIUIF, Moscow: Nauchno-Issled. Inst. Udobr. Insektofungits. im. Professora Ya. V. Samoilova, 2004.
Levin, B.V., et al., Topicality and practical steps toward the involvement of low-grade phosphate raw materials in processing into complex fertilizers, Khim. Prom-st. Segodnya, 2006, no. 11, pp. 11−18.
Petropavlovskii, I.A., Dmitrevskii, B.A., Levin, B.V., and Pochitalkina, I.A., Technology of Mineral Fertilizers: A Study Guide, St. Petersburg: Prospekt Nauki, 2018, p. 179.
Marcato, R. and Giulietti, M., Production of dicalcium phosphate by treatment of phosphate rock concentrate with nitric acid, Fert. Res., 1993, vol. 34, pp. 203−207.
Lur’e, Yu.Yu., Handbook of Analytical Chemistry, Moscow: Khimiya, 1989.
Karmyshov, V.F., et al., New forms of phosphorus-containing fertilizers and slow-release fertilizers, Obz. Inf. Ser. Obshcheotrasl. Vopr., 1984, no. 9(227).
Turaev, D.Yu. and Pochitalkina, I.A., RF Patent 2701320, A method to produce soluble phosphates of sodium, potassium, and ammonium (variants), Byull. Izobret., 2019, no. 27.
Turaev, D.Yu. and Pochitalkina, I.A., RF Patent 2701907, A method to produce mono-, di-, and tribasic phosphates of sodium, potassium, and ammonium, Byull. Izobret., 2019, no. 28.
Turaev, D.Yu. and Pochitalkina, I.A., RF Patent 2703777, A method to produce soluble orthophosphates, Byull. Izobret., 2019, no. 30.
Turaev, D.Yu. and Pochitalkina, I.A., RF Patent 2720285, A method to produce mono and dibasic calcium phosphates, Byull. Izobret., 2020, no. 13.
ACKNOWLEDGMENTS
We are grateful to the personnel of the Center for Collective Use, Mendeleev University of Chemical Technology of Russia, Moscow, Russia (analysis of samples by X-ray fluorescence analysis and scanning electron microscopy) and the personnel of the Center for Collective Use, Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Moscow, Russia (analysis of samples by X-ray powder diffraction) for their help with the performance of instrumental methods of analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Turaev, D.Y., Pochitalkina, I.A. Theoretical and Practical Foundations of the Selective Separation of Phosphate Ions from Phosphate Ores with a High Content of Iron Impurities with the Recirculation Method. Theor Found Chem Eng 56, 252–264 (2022). https://doi.org/10.1134/S0040579522020142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579522020142