Abstract
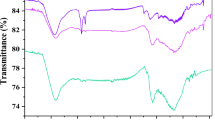
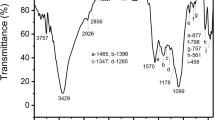
Effect of activating procedure on the physic-chemical properties and adsorption performance of activated carbon was investigated. For this, pomegranate peel was used as biomass and carbonized to prepare base material. Activating process was carried out using traditional (sulfuric acid as activating agent) and green way methods (water as activating agent). SEM, FTIR and particle size analysis were conducted to characterize as-prepared biosorbents. The adsorption capacity of carbonized pomegranate peel, acid-activated carbon and water-activated carbon were measured through separation of pyridine from aqueous solution. The effect of initial concentration and solution pH was also studied for all samples. Results depicted that water activated carbon achieved maximum value of adsorption capacity among three adsorbents at the other equal conditions. Additionally, kinetic studies were conducted to find predicting model for each adsorbents. According to the results, pseudo second order model can be applied for all three kinds of biosorbents at certain adsorption conditions.











Similar content being viewed by others
REFERENCES
Zalat, O.A. and Elsayed, M.A., A study on microwave removal of pyridine from wastewater, J. Environ. Chem. Eng., 2013, vol. 1, p. 137.
Kirk-Othmer Encyclopedia of Chemical Technology, Kroschwitz, J.I., Ed., New York: Wiley, 1987, 5th ed.
Lataye, D.H., Mishra, I.M., and Mall, I.D., Removal of pyridine from aqueous solution by adsorption on bagasse fly ash, Ind. Eng. Chem. Res., 2006, vol. 45, p. 3934.
Satyanarayana, T., Johri, B.N., and Prakash, A., Microorganisms in Environmental Management: Microbes and Environment, New York: Springer, 2012.
Padoley, K.V., Rajvaidya, A.S., Subbarao, T.V., and Pandey, R.A., Biodegradation of pyridine in a completely mixed activated sludge process, Bioresour. Technol., 2006, vol. 97, no. 10, p. 1225.
Sandhya, S., Urn, T.S., Stynarayana, S., and Kaul, S.N., Biodegradation of pyridine from pharmaceutical wastewater, Int. J. Environ. Stud., 2002, vol. 5, p. 1097.
Rhee, S.K., Lee, G.M., and Lee, S.T., Influence of a supplementary carbon source on biodegradation of pyridine by freely suspended and immobilized Pimelobacter sp., Appl. Microbiol. Biotechnol., 1996, vol. 44, no. 6, p. 816.
Lee, S.T., Rhee, S.K., and Lee, G.M., Biodegradation of pyridine by freely suspended and immobilized Pimelobacter sp., Appl. Microbiol. Biotechnol., 1994, vol. 41, no. 6, p. 652.
Stem, M., Heinzle, E., Kut, G.M., and Hungerbühler, K., Removal of substituted pyridines by combined ozonation/fluidized bed biofilm treatment, Water Sci. Technol., 1997, vol. 35, no. 4, p. 329. https://doi.org/10.1016/S0273-1223(97)00042-5
Tiana, F., Zhu, R., and Ouyang, F., Synergistic photocatalytic degradation of pyridine using precious metal supported TiO2 with KBrO3, J. Environ. Sci., 2013, vol. 25, no.11, p. 2299.
Akita, S. and Takeuchi, H., Sorption equilibria of pyridine derivatives in aqueous solution on porous resins and ion exchange resins, J. Chem. Eng. Jpn., 1993, vol. 26, no. 3, p. 237.
Mohan, D., Singh, K.P., Sinha, S., and Gosh, D., Removal of pyridine from aqueous solution using low cost activated carbons derived from agricultural waste materials, Carbon, 2004, vol. 42, p. 2409.
Zhao, B., Liang, H.D., Han, D.M., Qiu, D., and Chen, S.Q., Adsorption of pyridine from aqueous solution by surface treated carbon nanotubes, Sep. Sci. Technol., 2007, vol. 42, p. 3419.
Bai, Y., Sun, Q., Xing, R., Wen, D., and Tang, X., Removal of pyridine and quinoline by bio-zeolite composed of mixed degrading bacteria and modified zeolite, J. Hazard. Mater., 2010, vol. 181, p. 916.
Mahramanlioglu, M., Ozgen, O., Cinarli, A., and Kizilcikli, I., Adsorption of pyridine by acid treated spent bleaching earth, Asian J. Chem., 2010, vol. 22, no.2, p. 1428.
Gosu, V., Ram Gurjar, B., Surampalli, R.Y., and Zhang, T.C., Treatment of pyridine-bearing wastewater by Nano Zero-valent iron supported on activated carbon derived from agricultural waste, Desalin. Water Treat., 2016, vol. 57, p. 6250.
Lataye, D.H., Mishra, I.M., and Mall, I.D., Multicomponent sorption of pyridine and its derivatives from aqueous solution onto rice husk ash and granular activated carbon, Pract. Period. Hazard., Toxic, Radioact. Waste Manage., 2009, vol. 13, p. 218.
Zhu, Q., Moggridge, G.D., Ainte, M., Mantle, M.D., Gladden, L.F., and D’Agostino, C., Adsorption of pyridine from aqueous solutions by polymeric adsorbents MN 200 and MN 500. Part 1: Adsorption performance and PFG-NMR studies, Chem. Eng. J., 2016, vol. 306, p. 67.
Zhang, C., Chen, L., Wang, T.J., Su, C.L., and Jin, Y., Synthesis and properties of a magnetic core–shell composite nano-adsorbent for fluoride removal from drinking water, Appl. Surf. Sci., 2014, vol. 317, p. 552.
Rao, R.A.K. and Rehman, F., Adsorption of heavy metal ions on pomegranate (Punicagranatum) peel: Removal and recovery of Cr(VI) ions from a multi-metal ion system, Adsorpt. Sci. Technol., 2010, vol. 28, no. 3, p. 195.
Kumar, A. and Kumar, V., Kinetic, equilibrium isotherm and thermodynamic study for removal of cadmium from wastewater by using modified pomegranate peel, J. Chem. Pharm. Res., 2015, vol. 7, no. 1, p. 685.
Shouman, M.A. and Khedr, S.A.A., Removal of cationic dye from aqueous solutions by modified acid-treated pomegranate peels (PUNICA GRANATUM): Equilibrium and kinetic studies, Asian J. Appl. Sci., 2015, vol. 3, no. 4, p. 574.
Rohani Moghadam, M., Nasirizadeh, N., Dashti, Z., and Babanezhad, E., Removal of Fe(II) from aqueous solution using pomegranate peel carbon: Equilibrium and kinetic studies, Int. J. Ind. Chem., 2013, vol. 4, p. 19.
Hashim, A.Z.M., Sufian, S., and Shaharun, M.S., Green functionalization of activated carbon for dye removal application, Appl. Mech. Mater., 2015, vol. 755, p. 719.
Fisal, A., Wan Daud, W.M.A., Ahmad, M.A., and Radzi, R., The effects of acid leaching on porosity and surface functional groups of cocoa (The obroma cacao)-shell based activated carbon, Chem. Eng. Res. Des., 2013, vol. 90, p. 1.
Ramdane, N., Bouchelta, C., Marsa, Z., Medjram, M.S., and Magrib, P., Production of activated carbon from apple waste prepared under N2/microwave radiations, J. Chem. Pharm. Res., 2016, vol. 8, no. 1, p. 617.
Saka, C., BET, TG–DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2, J. Anal. Appl. Pyrolysis, 2012, vol. 95, p. 21.
Deng, H., Zhang, G., Xu, X., Tao, G., and Dai, J., Optimization of preparation of activated carbon from cotton stalk by microwave assisted phosphoric acid chemical activation, J. Hazard. Mater., 2010, vol. 182, p. 217.
Guigue, J., Mathieu, O., Lévêque, J., Mounier, S, Laffont, R., Maron, P.A., Navarro, N., Chateau, C., Amiotte-Suchet, P., and Lucas, Y., A comparison of extraction procedures for water-extractable organic matter in soils, Eur. J. Soil Sci., 2014, vol. 65, p. 520.
Khademzadeh Moghaddam, H. and Pakizeh, M., Experimental study on mercury ions removal from aqueous solution by MnO2/CNTs nanocomposite adsorbent, J. Ind. Eng. Chem., 2015, vol. 21, p. 221.
Alonso-Davila, P., Torres-Rivera, O.L., Leyva-Ramos, R., and Ocampo-Perez, R., Removal of pyridine from aqueous solution by adsorption on an activated carbon cloth, Clean: Soil, Air, Water, 2012, vol. 40, no. 1, p. 45.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahdieh Namvar-Mahboub, Ahsani, F. & Ansari, S. Preparation and Characterization of Nanosized Pomegranate Peel-Based Activated Carbon for Application in Pyridine Removal from Aqueous Solution. Theor Found Chem Eng 54, 940–948 (2020). https://doi.org/10.1134/S0040579520050371
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520050371