Abstract
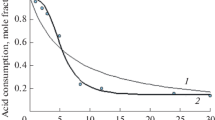
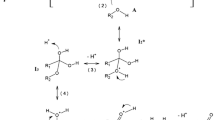
The reaction kinetics of esterification-hydrolysis in the n-propanol + acetic acid + n-propyl acetate + water system is considered. The phase state of the reaction mixture-homogeneous or two-phase-can change in the course of reaction due to the occurrence of a splitting region. For the analysis of the behavior of the system under these conditions, experimental data on the reaction kinetics were obtained at 293.15 K for both the homogeneous and heterogeneous regions of compositions. The evolution of the reaction system in time with the simultaneous occurrence of a chemical process and interphase mass transfer is also considered.
Similar content being viewed by others
References
Serafimov, L.A., Timofeev, S.V., Pisarenko, Yu.A., and Solokhin, A.V., Tekhnologiya osnovnogo organicheskogo sinteza: Sovmeshchennye protsessy (Basic Organic Synthesis Engineering: Combined Processes), Moscow: Khimiya, 1993.
Serafimov, L.A., Pisarenko, Y.A., and Kulov, N.N., Coupling chemical reaction with distillation: thermodynamic analysis and practical applications, Chem. Eng. Sci., 1999, vol. 54, pp. 1383–1388.
Pisarenko, Y.A., Serafimov, L.A., Cardona, C.A., Efremov, D.L., and Shuwalov, A.S., Reactive distillation design: analysis of the process statics, Rev. Chem. Eng., 2001, vol. 17, pp. 253–325.
Timofeev, V.S., Serafimov, L.A., and Timoshenko, A.V., Printsipy tekhnologii osnovnogo organicheskogo i neftekhimicheskogo sinteza (Principles of Basic Organic and Petrochemical Synthesis Engineering), Moscow: Vysshaya Shkola, 2010.
Serafimov, L.A. and Frolkova, A.K., Fundamental principle of concentration-field redistribution between separation regions as a basis for the design of technological systems, Theor. Found. Chem. Eng., 1997, vol. 31, pp. 159–166.
Balashov, M.I., Physicochemical foundations and technological principles of the organization of reactive distillation processes, Extended Abstract of Doctoral (Eng.) Dissertation, Moscow: Moscow Inst. of Fine Chemical Technology, 1980.
Pisarenko, Yu.A., Development of theoretical foundations of analysis of steady-state regimes of reactionmass transfer processes, Extended Abstract of Doctoral (Eng.) Dissertation, Moscow: Moscow State Academy of Fine Chemical Technologies, 1997.
Zharov, V.T. and Pervukhin, O.K., Structure diagram of liquid-vapor equilibrium in a system with chemical interaction. 2. Methanol formic acid methylformatewater system, Zh. Fiz. Khim., 1972, vol. 46, pp. 1970–1973.
Gorovits, B.I., Toikka, A.M., Pisarenko, Yu.A., and Serafimov, L.A., Thermodynamics of heterogeneous systems with chemical interaction, Theor. Found. Chem. Eng., 2006, vol. 40, pp. 239–244.
Toikka, A.M., Toikka, M.A., Pisarenko, Yu.A., and Serafimov, L.A., Vapor-liquid equilibria in systems with etherification reaction, Theor. Found. Chem. Eng., 2009, vol. 43, pp. 129–142.
Pervukhin, O.K., Certain problems of thermodynamics of open systems with chemical-reaction, Zh. Fiz. Khim., 1991, vol. 65, pp. 2891–2899.
Zharov, V.T., On the thermodynamics of liquid-vapor equilibrium and evaporation in reactive systems, in Voprosy termodinamiki geterogennykh sistem i teorii poverkhnostnykh yavlenii (Topics in the Thermodynamics of Heterogeneous Systems and Surface Effect Theory), Storonkin, A.V. and Zharov, V.T., Eds., Leningrad: Leningr. Gos. Univ., 1973.
Doraiswamy, L., Organic Synthesis Engineering New York: Oxford Univ. Press, 2001.
Wasewar, K.L., Pangarkar, V.G., Heesink, A.B.M., and Versteeg, G.F., Intensification of enzymatic conversion of glucose to lactic acid by reactive extraction, Chem. Eng. Sci., 2003, vol. 58, pp. 3385–3393.
Wasewar, K.L., Heesink, A.B.M., Versteeg, G.F., and Pangarkar, V.G., Equilibria and kinetics for reactive extraction of lactic acid using Alamine 336 in decanol, J. Chem. Technol. Biotechnol., 2002, vol. 77, pp. 1068–1075.
Chong, M.F., Chen, J., Oh, P.P., and Chen, Z.-S., Modeling study of chemical phase equilibrium of canola oil transesterification in a CSTR, Chem. Eng. Sci., 2013, vol. 87, pp. 371–380.
Toikka, M.A., Liquid-liquid equilibrium, critical states, and chemical equilibrium in separating reactive systems, Cand. Sci. (Chem.) Dissertation, St. Petersburg: St. Petersburg State Univ., 2010.
Kocherbitov, V.V. and Toikka, A.M., Liquid-vapor equilibrium in the acetic acid-n-propyl alcohol-water system at 313.15 K, Russ. J. Appl. Chem., 1997, vol. 70, pp. 1691–1695.
Toikka A.M., Gorban, Y.P., Ivanova, Z.V., and Kocherbitov, V.V., Liquid-vapor, liquid-liquid, and liquidliquid-vapor phase equilibria in water-acetic acidpropyl acetate system at 313.15 K, Russ. J. Appl. Chem., 1997, vol. 70, pp. 41–43.
Kocherbitov, V.V. and Toikka, A.M., Liquid-vapor and liquid-liquid phase equilibria in the system acetic acid-n-propanol-water-n-propyl acetate at 313.15 K, Russ. J. Appl. Chem., 1999, vol. 72, pp. 1706–1708.
Toikka, M.A., Gorovits, B.I., and Toikka, A.M., Solubility in the system constituted by acetic acid, n-propanol, water, and n-propyl acetate, Russ. J. Appl. Chem., 2008, vol. 81, pp. 223–230.
Toikka, M.A., Tsvetov, N.S., and Toikka, A.M., Splitting of the liquid solution and the compositions of liquid phases in the water-n-propanol-n-propyl acetate system at 293.15, 303.15, and 313.15 K, Theor. Found. Chem. Eng., 2011, vol. 45, pp. 429–435.
Toikka, M.A., Tsvetov, N.S., and Toikka, A.M., Experimental study of chemical equilibrium and vapor-liquid equilibrium calculation for chemical-equilibrium states of the n-propanol-acetic acid-n-propyl acetate-water system, Theor. Found. Chem. Eng., 2013, vol. 47, pp. 554–562.
Bart, H., Kaltenbrunner, W., and Landschutzer, H., Kinetics of esterification of acetic acid with propyl alcohol by heterogeneous catalysis, Int. J. Chem. Kinet., 1996, vol. 28, pp. 649–656.
Tsvetov, N.S., Pervukhin, O.K., and Toikka, A.M., Esterification kinetics in the n-propanol-acetic acid-n-propyl acetate-water system in the liquid-liquid separation region at 293.15 K, Vestn. S.-Peterburg. Gos. Univ., Ser. 4: Fiz., Khim., 2014, no. 1, pp. 84–93.
Emanuel’, N.M. and Knorre, D.G., Kurs khimicheskoi kinetiki (Chemical Kinetics), Moscow: Vysshaya Shkola, 1984.
NIST Chemistry WebBook. NIST Standard Reference Database, No. 69, Linstrom, P.J. and Mallard, W.G., Eds., Gaithersburg, Md.: National Institute of Standards and Technology http://webbook.nist.gov
Huang, Y.-S., Sundmacher, K., Tulashie, S., and Schlunder, E.-U., Theoretical and experimental study on residue curve maps of propyl acetate synthesis reaction, Chem. Eng. Sci., 2005, vol. 60, pp. 3363–3371.
Wyczesany, A., Chemical equilibrium constants in esterification of acetic acid with C1-C5 alcohols in the liquid phase, Chem. Process Eng., 2009, vol. 30, pp. 243–265.
Ingold, G.K., Structure and Mechanism in Organic Chemistry, London: Cornell Univ., 1969, 2nd ed.
Belousov, V.P. and Panov, M.Yu., Termodinamika vodnykh rastvorov neelektrolitov (Thermodynamics of Aqueous Nonelectrolyte Solutions), Moscow: Khimiya, 1983.
Piiskop, S., Hagu, H., Jarv, J., Salmar, S., and Tuulmets, A., Sonification effects on ester hydrolysis in alcohol-water mixtures, Proc. Estonian Acad. Sci. Chem., 2007, vol. 56, pp. 199–206.
Van’t Hoff, J.H., études de dynamique chimique, Amsterdam: Mü, 1884.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.S. Tsvetov, O.K. Pervukhin, A.M. Toikka, 2015, published in Teoreticheskie Osnovy Khimicheskoi Tekhnologii, 2015, Vol. 49, No. 3, pp. 312–321.
Rights and permissions
About this article
Cite this article
Tsvetov, N.S., Pervukhin, O.K. & Toikka, A.M. Kinetics of esterification and hydrolysis in the n-propanol + acetic acid + n-propyl acetate + water system in homogeneous and heterogeneous composition areas. Theor Found Chem Eng 49, 297–305 (2015). https://doi.org/10.1134/S0040579515030148
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579515030148