Abstract
Solid solution \({\text{G}}{{{\text{d}}}_{{2 - x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7 - x}}}\) with a pyrochlore structure is synthesized for the first time. The cationic composition is confirmed via chemical analysis and nuclear reactions. It is found that the stoichiometry with respect to lithium is retained up to 1100°C. The lattice parameter diminishes in the homogeneity range 0 ≤ x ≤ 0.30, while the free volume of migration grows. Introducing lithium into the Gd sublattice raises oxygen–ion conductivity, due to the emergence of oxygen vacancies and enhancement of their mobility. Maximum conductivity is reached for composition with х = 0.10 (~1 × 10−3 Ω−1 cm−1, 650°C). An assumption is made about the formation of associates of the type \({{\{ {\text{Li}}_{{{\text{Gd}}}}^{{''}} \cdot {\text{V}}_{{\text{o}}}^{{ \bullet \bullet }}\} }^{ \times }}\) at high contents of the dopant (x = 0.30), accompanied by an increase in the activation energy of conductivity.
Similar content being viewed by others
INTRODUCTION
The use of molten salt electrolytes opens up new resource-saving ways of processing industrial waste, including radioactive substances and organic compounds, that are safe for humans and the environment [1–3]. Electrochemical ways present minimal environmental hazards and allow us to create controlled closed technological schemes. Impurities (mainly of oxygen) contained in them have a strong effect on the properties of molten salt electrolytes. Reliable oxygen sensors are therefore needed in particular for measuring both contents of oxygen in situ during chemical processes and those in technological media [4]. However, this problem remains unsolved. Materials currently proposed for oxygen ion activity sensors are susceptible to corrosion in melts based on halides. They are thus ineffective, since they cannot ensure accurate control over the parameters of technological processes [5, 6].
Crystal matrix Gd2Zr2O7 with a pyrochlore structure is of interest as a material that could be used as an O2– sensor for Li+-halide melts. Gadolinium zirconate Gd2Zr2O7 has attracted considerable attention from scientists in the last few years, due to its high thermochemical stability and structural flexibility [7, 8]. There have recently been intense studies of the transport properties of phases based on Ln2Zr2O7, including Gd2Zr2O7. The transport of oxygen ions in pyrochlores of general formula A2B2O (1)6O (2) is due to the possibility of anti-Frenkel disordering (i.e., oxygen vacancies at 48f and interstitial oxygen at 8b) [9, 10]. Acceptor doping of matrix Ln2Zr2O7 is usually used to identify oxygen deficiencies and improve O2– ion conduction. It was found for phase Gd2Zr2O7 that isovalent substitutions of Gd3+ with La3+, Er3+, Y3+, Dy3+, Sm3+, and Nd3+ had no appreciable effect on ionic conductivity [11–13]. The highest conductivity was observed for samples with Gd3+ sublattices doped with doubly charged cations [14–16].
In terms of possibly using such phases as sensors on O2– ions in melts containing Li+, we must also preclude the possibility of ion exchange in the melt, so it is advisable to use Li+.
In this work, solid solution \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) is synthesized for the first time. The width of the homogeneity range is evaluated, transport properties are investigated, and the effects the nature and concentration of defects have on oxygen-ion transport are discussed.
EXPERIMENTAL
Samples of \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) (x = 0.10, 0.20, 0.25, 0.30, 0.33, 0.35, 0.40, 0.45, 0.50, 0.60) were obtained using a modified Pechini approach and glycerol-nitrate technology. Corresponding weights of pre-calcined powders of Gd2O3 (GdO-D, TU 48-4-524-90) (600°C, 3 h) and Li2CO3 (special purity, TU 6-09-4757-84) (400°C, 2 h) were dissolved in an excess of concentrated nitric acid (special purity 27-5, Russia) at 60–80°C. The content of crystallization water was preliminarily determined via thermogravimetry for zirconyl nitrate ZrO(NO3)2⋅nH2O (analytical grade, TU 6-09-1406-76, Russia), and a corresponding amount was dissolved in distilled water. The concentration of the resulting solutions ranged from ~0.5 to 1 mol/L. All solutions were then mixed together. Citric acid (extra pure grade, TU 6-09-584-75), used as a ligand in complexation reactions, and glycerol (analytical grade, GOST 6259-75, VEKTON), which serves as a polymer-forming agent, were next added with stirring. The molar ratio of the sum of metal cations to citric acid and glycerol was 1 : 1.5 : 1. An excess of citric acid in a ratio of 1.5 mol for each mol of metal ion was taken to create the necessary conditions for the formation of possible polynuclear citrate complexes. The resulting mixture was neutralized to pH 4 with a solution of ammonia. The mixture was then heated for several hours at temperatures of 80–90°C with the removal of nitrogen oxides. Heating also resulted in the removal of water and initiation of the polycondensation of citrate complexes and an excess of citric acid with glycerol. Polycondensation produced a gel that was subjected to stepwise drying at temperatures of 100 to 140°C. Heat treatment was done for several hours at 400°C to remove residual organic compounds and decompose organometallic compounds.
The main strategy for removing the organic matrix was maintaining homogeneity (which ensured the uniformity of the distribution of elements in the final product) and preventing deviation from stoichiometry with respect to lithium, due to the volatility of its oxide. These requirements were met by the proposed heat treatment scheme, which ensured a smooth flow of processes. There was no spontaneous combustion of the solutions that boiled off, but lithium was bound into intermediate phases.
To form the final product, heat treatment was performed in the temperature range of 700–1100°C with steps of 100°C and holding for 12 h at each stage, with intermediate grinding in ethanol in an agate mortar. The last stage of synthesis (1100°C, 20 h) was performed by heating in a laboratory microwave oven (Ural-Gefest, Russia) and coincided with the temperature of ceramic sintering.
X-ray analysis was performed to control the phase composition of the products of synthesis and determine the structural parameters of the considered samples. X-ray diffraction patterns were obtained at room temperature on a Bruker D8 Advance diffractometer (Germany) in the range of angles 2θ = 10°–90° with steps of 0.01° and exposition of 1 s at each point. The interplanar distances were calculated and the parameters of unit cells were determined using the FullProf program.
The surface morphology of the samples was studied on a Phenom ProX scanning electron microscope (Eindhoven, The Netherlands). The tablets were not subjected to any special treatment for analysis. Images were recorded at 15 kV and a working distance of 10 mm using a back scattering detector. The operation of the microscope was monitored, and the operating parameters were altered using the Phenom Pro Suite software.
Samples of \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) were analyzed in a number of ways to determine its composition.
Chemical analysis was performed for gadolinium and zirconium after annealing at each temperature via inductively coupled plasma atomic emission spectroscopy on an Optima 4300 DV Perkin Elmer spectrometer (United States). A 0.1 g sample was preliminarily dissolved in a mixture of concentrated sulfuric acid and ammonium sulfate with a ratio of 3 : 2 by weight.
The content of lithium was determined via nuclear microanalysis using the reaction 7Li(p, α)4He. The source of the accelerated protons was a 2 MV Van de Graaff accelerator, and the particle energy of the initial beam was 762 keV. Nuclear reaction spectra were recorded using a silicon surface-barrier detector with an area of 1 cm2. The irradiation of the samples was measured using a secondary monitor with an error of 1% for lithium. Powder particles were pressed into an indium plate to make measurements via nuclear microanalysis. A layer at least 2 μm thick and containing only oxide particles formed near the outer surface of each sample. To calculate the concentrations of lithium, we compared spectra from the test sample and a reference sample with a concentration of lithium that was constant over depth. The reference for lithium was Li2ZrO3, the content of lithium in which was taken to be 33.33 at % (9.06 wt %) in accordance with the stoichiometric formula. Since the concentration of lithium isotope 7Li was determined and its content was 92.5% in the natural mixture of isotopes, the obtained values must be divided by 0.925 to determine the concentration of lithium. This allowed us to determine the concentration of lithium with a high degree of accuracy, due to the large cross section of the nuclear reaction on isotope 7Li when a sample was irradiated with protons. The root-mean-square error in measuring the lithium concentration in the studied samples did not exceed 8%.
For electrical measurements, the powders were pressed into pellets ~10 mm in diameter and ~2 mm thick. The compression and sintering of powders was done in a number of ways.
• Spark-plasma sintering on a hybrid SPS Labox-125 setup (Japan). Heating was done by passing a pulsed direct current at a rate of 150 and 200 K/min up to 1100°C. The temperature was controlled outside the graphite matrix using an optical pyrometer and a thermocouple. The powder was compacted with a constant load (at a pressure of 5, 25, 50 MPa) before heating. The load was removed after heating was stopped. The high temperature holding time was 60 min, at the end of which both the sample and the chamber were cooled at a rate of 10 K/min.
• Using a hand press with a pressure of ~6.5 MPa. The pressed samples were sintered inside a microwave oven for 20 h at 1100°C.
The density of ceramic samples was measured according to their geometric dimensions and via hydrostatic weighing in kerosene, in accordance with GOST 2409-2014.
Electrodes made of fine platinum (platinum paste in a solution of rosin in ethanol) were applied to the end surfaces of the sintered samples to measure electrical conductivity. The organic binder was burned out and the Pt electrodes were baked for 1 h at a temperature of 900°C. The electrical conductivity of the studied samples was measured according to the electrochemical impedance in a two-contact cell, using a Z‑500PX impedance parameter meter (Elins, Chernogolovka). The frequency range of measurements was 100 Hz–1 MHz at temperatures of 300–900°C. Resistance was determined by extrapolating the impedance spectrum to the axis of active resistance using the ZView2 software. Specific electrical conductivity was found with the familiar ratio
where R is the sample’s resistance, l is its thickness, and S is its cross-section area. Electrical conductivity was measured in air.
RESULTS AND DISCUSSION
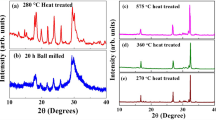
X-ray data showed that the samples of \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) in the range of compositions 0 ≤ x ≤ 0.30 consisted of a single phase characterized by a pyrochlore structure (sp.gr. Fd\(\bar {3}\)m) along with the basic composition of Gd2Zr2O7. A slight broadening of diffraction lines and the emergence of a shoulder were observed on the X-ray diffraction patterns of samples with x = 0.33 and 0.35, as is shown in the inset in Fig. 1 for the example of composition x = 0.35. It was not possible to reliably identify the nature of the second phase for these compositions. Samples with high lithium contents of x ≥ 0.40 contained an impurity phase of lithium zirconate Li2ZrO3. X-ray diffraction patterns of the synthesized samples of \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) are shown in Fig. 1.
When lithium was introduced into Gd3+ sublattice, the lattice parameter fell monotonically (Fig. 2), which agrees with the ratio of the radii of substituting atoms: (r(Gd3+) = 1.053 Å, r(Li+) = 0.92 Å for coordination number of 8 [17]).
According to data from scanning electron microscopy of the lithium-doped materials, the shape and dispersion of grains were approximately the same for each of the studied samples, which consisted of rounded particles with sizes of 3–5 µm. However, the emergence of individual uniformly distributed grains of different colors was observed for samples of composition x ≥ 0.40, suggesting the presence of an impurity phase (Fig. 3). According to energy dispersive X-ray microanalysis, the main component of this phase was ZrO2, so the impurity phase was obviously lithium zirconate Li2ZrO3 (since it is a light element, lithium was not detected). We may therefore conclude that the homogeneity range of the studied solid solution was limited by the composition x = 0.30.
The chemical composition of the obtained phases was monitored at all stages of heat treatment during synthesis and in preparing the ceramic samples. No deviations from stoichiometry were observed for gadolinium and zirconium; their atomic ratios did not change and remained in exact correspondence with the charged samples. The lithium content was maintained only up to a temperature of 1100°C. There was a considerable loss of lithium upon annealing at higher temperatures, which is described in Table 1 using the example of Gd1.7Li0.3Zr2O6.7.
Because of the above, our task was to obtain ceramics of a given stoichiometry with respect to lithium. Means of low-temperature sintering (spark plasma and microwave) were therefore used. Ceramics obtained via spark plasma sintering had a relative density of 70%. According to data on nuclear reactions, however, there was a redistribution of the lithium content: an increase in 6.75 times relative to the nominal composition was observed on the surface of a pellet, accompanied by a reduction in its volume. Other ceramics were therefore obtained via 20 h of microwave sintering at 1100°C, allowing us to preserve the stoichiometry with respect to lithium. The samples were characterized by a relative density of ~70%.
Electrical Measurements
It is known that the electrical properties of polycrystalline solid electrolytes must be compared using samples of approximately the same relative density, since their electrical conductivity depends on the porosity of the material [18]. Electrical conductivity is therefore normally measured on high-density samples. Since high-temperature annealing was not performed in order to preserve the stoichiometry of solid solution \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) with respect to lithium and the relative density was ~70%, data on the electrical conductivity of samples with the same relative density were used for a correct comparison to matrix phase Gd2Zr2O7. The electrical conductivities of Gd2Zr2O7 samples with different relative densities were presented in [19].
Temperature dependences of \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) electrical conductivity are compared in Fig. 4. We can see that introducing the dopant did not alter the general nature of the dependences, but the conductivity changed dramatically. The oxygen-ion character of the conductivity of Gd2Zr2O7 and doped phases based on it was proved in [19].
The concentration dependence of conductivity is shown in Fig. 5. We can see that the electrical conductivity rose along with the concentration of lithium, a natural consequence of the increased concentration of oxygen vacancies upon doping.
The formation of oxygen vacancies upon introducing lithium into the gadolinium sublattice is described by the quasi-chemical formalism of the reaction
Reaching its maximum value for composition with x = 0.10, conductivity then falls. This behavior is a consequence of several multidirectional factors. Ion transfer in solid electrolytes is known to be determined by the concentration of mobile ions and their charge and mobility. The last is in turn determined by the size of the ion, the number of crystallographic positions available to mobile ions, the energy of a mobile ion bonding with the crystal lattice, and the geometry of the unit cell [20].
The concentration of oxygen vacancies rises upon the doping of Gd2Zr2O7 with lithium, which favors an increase in oxygen-ion conductivity. However, the lattice is compressed. Ionic conductivity is known to be proportional to the mean free path, and hence to the interatomic distance. An increase in the lattice parameter therefore facilitates ion transport. As can be seen from the data in Table 2, the preexponential factor in the equation
falls as the concentration of lithium grows (i.e., with a drop in the lattice parameter, which is consistent with the above reasoning).
On the other hand, doping increases the free volume of migration (Table 2), which facilitates the migration of oxygen ions. It should also be noted that the nature of the dependence of the activation energy (a quantity that directly reflects ion mobility) confirms the above. Activation energies of ionic conductivity Ea, calculated according to Eq. (3) and depending on the content of the dopant, are also shown in Table 2. We can see that the activation energy falls upon an increase in the lithium content.
At the same time, it is known that the conductivity of many oxygen-ion conductors depend strongly on the concentration of the dopant [21]. In such systems, a drop in conductivity in the region of high concentrations of an acceptor-type dopant is usually explained by effects of the interaction between defects with opposite charges [21]. We may assume that typical patterns of oxygen-ion transport are observed for the considered \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) system (i.e., associates of the \({{\{ {\text{Li}}_{{{\text{Gd}}}}^{{''}} \cdot {\text{V}}_{{\text{o}}}^{{ \bullet \bullet }}\} }^{ \times }}\) type can form at high concentrations of defects). This lowers the concentration of free oxygen vacancies and reduces conductivity. The data in Table 2 for a sample with high lithium content x = 0.30 show that the activation energy is higher than for other Li+-doped phases, confirming our assumption about the association of defects.
Introducing lithium into the Gd3+ sublattice thus allows us to obtain solid solution \({\text{G}}{{{\text{d}}}_{{2 - x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7 - x}}}\) in a fairly wide range of concentrations (0 ≤ x ≤ 0.30). There is an optimum range of dopant concentrations for which oxygen-ion conductivity reaches its maximum and exceeds the values of Gd2Zr2O7 by an order of magnitude. We may therefore assume that lithium is an effective acceptor dopant for the pyrochlore structure.
CONCLUSIONS
Solid solutions with pyrochlore structure \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) were obtained using a modified Pechini approach. Monitoring the content of lithium in a sample with a method employing nuclear reactions allowed us to develop ways of synthesis that preserve the stoichiometry with respect to lithium. Phase Gd2Zr2O7 displayed high tolerance to lithium as a dopant, and was characterized by a fairly wide homogeneity range of \({\text{G}}{{{\text{d}}}_{{2-x}}}{\text{L}}{{{\text{i}}}_{x}}{\text{Z}}{{{\text{r}}}_{2}}{{{\text{O}}}_{{7-x}}}\) solid solution (0 ≤ x ≤ 0.30). Introducing lithium into the Gd sublattice lowered the lattice parameter. Oxygen-ion conductivity can be raised by an order of magnitude (x = 0.10) with lithium doping, due to an increase in the concentration of oxygen vacancies and their mobility.
REFERENCES
A. Merwin, M. A. Williamson, et al., J. Electrochem. Soc. 164, H5236 (2017). https://doi.org/10.1149/2.0251708jes
E. Y. Choi and J. Lee, J. Nucl. Mater. 494, 439 (2017). https://doi.org/10.1016/j.jnucmat.2017.07.036
J. L. Willit, W. E. Miller, and J. E. Battles, J. Nucl. Mater. 195, 229 (1992). https://doi.org/10.1016/0022-3115(92)90515-M
E. Y. Choi, I. K. Choi, J. M. Hur, et al., Electrochem. Solid-State Lett. 15, E11 (2012). https://doi.org/10.1149/2.016203esl
S. H. Cho, D. Y. Kim, S. Kwon, et al., J. Nucl. Sci. Technol. 55, 97 (2018). https://doi.org/10.1080/00223131.2017.1383214
S. H. Cho, S. W. Kim, D. Y. Kim, et al., J. Nucl. Mater. 490, 85 (2017). https://doi.org/10.1016/j.jnucmat.2017.04.012
H. Lehmann, D. Pitzer, G. Pracht, et al., J. Am. Ceram. Soc. 86, 1338 (2003). https://doi.org/10.1111/j.1151-2916.2003.tb03473.x
A. V. Radha, S. V. Ushakov, and A. Navrotsky, J. Mater. Res. 24, 3350 (2009). https://doi.org/10.1557/jmr.2009.0401
A. J. Burggraaf, T. van Dijk, and M. J. Verkerk, Solid State Ionics 5, 519 (1981). https://doi.org/10.1016/0167-2738(81)90306-4
M. P. van Djik, A. J. Burggraaf, et al., Solid State Ionics 17, 159 (1985). https://doi.org/10.1016/0167-2738(85)90067-0
B. P. Mandal, S. K. Deshpande, and A. K. Tyagi, J. Mater. Res. 23, 911 (2008). https://doi.org/10.1557/jmr.2008.0112
J. A. Díaz-Guillen, M. R. Díaz-Guillen, J. M. Almanza, et al., J. Phys.: Condens. Matter 19, 356212 (2007). https://doi.org/10.1088/0953-8984/19/35/356212
J. A. Díaz-Guillen, A. F. Fuentes, M. R. Díaz-Guillen, et al., J. Power Sources 186, 349 (2009). https://doi.org/10.1016/j.jpowsour.2008.09.106
A. N. Radhakrishnan, P. P. Rao, K. S. M. Linsa, et al., Dalton Trans. 40, 3839 (2011). https://doi.org/10.1039/C0DT01688H
K. V. G. Kutty, C. Mathews, et al., Solid State Ionics 22, 99 (1995). https://doi.org/10.1016/0167-2738(95)00127-R
A. V. Shlyakhtina, Extended Abstract of Doctoral (Chem.) Dissertation (Moscow, 2010).
R. D. Shannon, Acta Crystallogr., A 32, 751 (1976). https://doi.org/10.1107/S0567739476001551
H. El. Khal, A. Cordier, N. Batis, et al., Solid State Ionics 304, 75 (2017). https://doi.org/10.1016/j.ssi.2017.03.028
I. A. Anokhina, I. E. Animitsa, V. I. Voronin, et al., Ceram. Int. 47, 1949 (2021).https://doi.org/10.1016/j.ceramint.2020.09.025
V. N. Chebotin and M. V. Perfil’ev, Electrochemistry of Solid Electrolytes (Khimiya, Moscow, 1978) [in Russian].
S. J. Skinner and J. A. Kilner, Mater. Today 6, 30 (2003). https://doi.org/10.1016/S1369-7021(03)00332-8
Funding
This work was performed with a grant from the Russian Foundation for Basic Research, project no. 20-43-660033, and financial support from the State Atomic Energy Corporation ROSATOM (State Contract no. Н.4о.241.19.21.1070 of April 16, 2021; electronic identifier 07731000003210000330001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anokhina, I.A., Animitsa, I.E., Voronin, V.I. et al. Electrical Properties of Li+-Substituted Solid Solutions Based on Gd2Zr2O7. Russ. J. Phys. Chem. 95, 2426–2431 (2021). https://doi.org/10.1134/S0036024421120037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421120037