Abstract
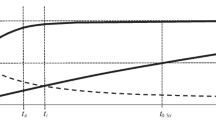
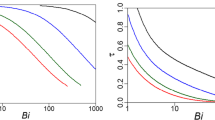
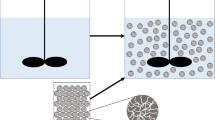
The possibility of deriving a model of the kinetics of batch sorption processes is considered by transforming a nonlinear difference kinetic equation with a nonconstant equilibrium parameter for the interface into approximate pseudo first- and second-order equations with fixed parameters, depending on the characteristic of equilibrium isotherms in a certain way. The calculated and experimental equilibrium and kinetic parameters are compared for a large number of processes, including the sorption and electrosorption of metal ions on activated coals, the sorption of lead on nanostructured carbon materials, and phosphatidylcholine sorption on ordered mesoporous nanostructured silica materials. Good coincidence is observed between the calculated equilibrium data and the respective experimental results obtained in kinetic experiments. At the same time, there is no correspondence between calculated and experimental dimensionless parameters based on the relationships of kinetic coefficients for different order equations (due apparently to great errors in determining these coefficients when processing the experimental data using the pseudo second-order model of kinetics). The obtained results confirm earlier conclusions that it is impossible to characterize the mechanisms of kinetic processes based only on good descriptions of experimental results using pseudo first- or second-order kinetic equations.

Similar content being viewed by others
REFERENCES
E. V. Venitsianov and R. N. Rubinshtein, Dynamics of Sorption from Liquid Media (Nauka, Moscow, 1983) [in Russian].
D. Muraviev, R. Kh. Khamizov, N. A. Tikhonov, and V. V. Kirshin, Langmuir 13, 7186 (1997).
D. Muraviev, R. Kh. Khamizov, and N. A. Tikhonov, Langmuir 19, 10852 (2003).
Y. Liu and L. Shen, Langmuir 24, 11625 (2008).
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918).
Y. S. Ho and G. McKay, Process Biochem. 34, 451 (1999).
Y. S. Ho and G. McKay, Water Res. 34, 735 (2000).
A. Ebadi, Adsorption, No. 15, 65 (2009).
D. A. Sveshnikova, K. G. Kunzhueva, D. R. Ataev, et al., Sorbtsion. Khromatogr. Protsessy 12, 789 (2012).
D. A. Sveshnikova, R. Kh. Khamizov, K. Sh. Rabadanov, et al., Sorbtsion. Khromatogr. Protsessy 15, 478 (2015).
A. E. Kucherova, Cand. Sci. (Tech. Sci.) Dissertation (Tambov State Tech. Univ., Tambov, 2016).
A. E. Kucherova, A. V. Gerasimova, A. E. Burakov, et al., Vestn. Tambov. Tekh. Univ. 22, 439 (2016).
A. E. Burakov, I. V. Romantsova, A. E. Kucherova, and A. G. Tkachev, Adsorption Sci. Technol. 32, 737 (2014).
A. E. Burakov, I. V. Romantsova, A. E. Kucherova, and A. G. Tkachev, Prot. Met. Phys. Chem. Surf. 51, 505 (2015).
O. V. Belousov, V. A. Parfenov, L. A. Solov’ev, and S. D. Kirik, RF Patent No. 2287485 (2006).
V. Meynen, Microporous Mesoporous Mater. 125, 170 (2009).
L. A. Sinyaeva, S. I. Karpov, N. A. Belanova, and F. Roessner, Sorbtsion. Khromatogr. Protsessy 16, 280 (2016).
A. S. Askurava, L. A. Sinyaeva, N. A. Belanova, S. I. Karpov, and F. Roessner, Sorbtsion. Khromatogr. Protsessy 16, 280 (2016).
O. O. Krizhanovskaya, L. A. Sinyaeva, S. I. Karpov, V. F. Selemenev, et al., Sorbtsion. Khromatogr. Protsessy 14, 784 (2014).
L. A. Sinyaeva, Cand. Sci. (Chem.) Dissertation (Voronezh State Univ., Voronezh, 2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Chernikova
Rights and permissions
About this article
Cite this article
Khamizov, R.K., Sveshnikova, D.A., Kucherova, A.E. et al. Kinetic Model of Batch Sorption Processes: Comparing Calculated and Experimental Data. Russ. J. Phys. Chem. 92, 2032–2038 (2018). https://doi.org/10.1134/S0036024418100114
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418100114